The Efficacy of XHANCE
- ReOpen1 & ReOpen2
Overview & Study Design - ReOpen1 & ReOpen2 Coprimary Endpoints
- ReOpen1 & ReOpen2 Acute Exacerbations
- ReOpen1 & ReOpen2 SNOT-22 Scores
- NAVIGATE II Study
Study design of trials
ReOpen1 and ReOpen2 are phase 3, double-blinded trials assessing XHANCE 186 mcg or 372 mcg (1 or 2 sprays/nostril) twice daily (BID) vs EDS-placebo. ReOpen1 (N=332) included patients with or without nasal polyps, whereas ReOpen2 (N=223) excluded patients with nasal polyps. The objective of both was to assess the efficacy of XHANCE for chronic rhinosinusitis (irrespective of nasal polyps).1,2
Polyps extending beyond the attachment of the inferior turbinate
Any observed polyps at all, defined as more than grade 0
(Patient Global Impression of Change [PGIC]) at Week 24
| Inclusion Criteria2 | Exclusion Criteria2 | ||
|---|---|---|---|
| 18 years or older | Septal perforation | ||
| Diagnosis of chronic rhinosinusitis consistent with guidelines* | Sino-nasal surgery within 6 months | ||
| Confirmatory CT evidence of sinus disease† | ReOpen1 Polyps extending beyond the attachment of the inferior turbinate |
ReOpen2 Any observed polyps at all, defined as more than grade 0 |
|
| Coprimary Endpoints2 | Secondary Endpoints2 | ||
|---|---|---|---|
| Change from baseline through Week 4 in Composite Symptom Score (CSS)‡ | Composite and individual symptom scores over time, through Week 12 | ||
| Change from baseline to Week 24 in sinus opacification1,3§ | Disease-specific quality of life (QoL) (22-item Sino-Nasal Outcome Test [SNOT-22] total score) at Week 24 | ||
| Patient-perceived global change in disease (Patient Global Impression of Change [PGIC]) at Week 24 |
- CSS change through Week 4 for patients who were symptomatic (met entry criteria) despite using a standard-delivery nasal steroid at trial entry
- Frequency of acute exacerbations||
†As indicated by 1 or more sinus on each side with Lund-Mackay score of 1 or more, combined ≥25% opacification of the ethmoid sinuses, and ≥25% opacification of at least 1 maxillary sinus.2
‡CSS: Summed congestion, facial pain/pressure, and nasal discharge scores, each 0=no symptoms to 3=severe in the morning (AM): averaged over previous 7 days.2
§As measured by the average of the percentages of opacified volumes in ethmoid and maxillary sinuses by CT scan.2
||Acute exacerbations of chronic rhinosinusitis were defined as (1) acute worsening of 1 or more cardinal symptoms of chronic rhinosinusitis lasting at least 3 days, and (2) escalation of care, defined as initiation of antibiotics or oral steroids, or an unscheduled acute care visit or inpatient care for increased symptoms of chronic or acute sinusitis.2
More information on XHANCE pivotal trials

Review details of ReOpen1 and ReOpen2 in the Journal of Allergy and Clinical Immunology: In Practice

Review details of NAVIGATE I and NAVIGATE II in the Journal of Allergy and Clinical Immunology
NOTE: These scientific publications contain additional outcome measures and other information, including exploratory endpoints, not contained in the full Prescribing Information, which are subject to important limitations.
XHANCE significantly improved chronic sinusitis symptoms for patients without nasal polyps1
Reduced symptoms were observed during a randomized, double-blind, placebo-controlled, parallel-group, multicenter study1
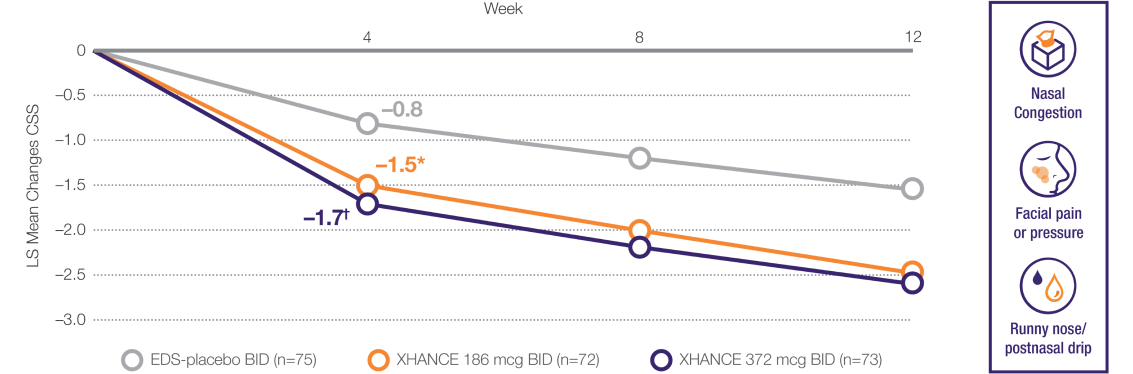
Coprimary Endpoint: Change From Baseline to Week 4 in Composite Symptom Score (CSS) in ReOpen2 (Without Nasal Polyps)2
*P≤0.05 vs EDS-placebo.2
†P≤0.001 vs EDS-placebo.2
BID=twice daily.
CSS: Summed congestion, facial pain/pressure, and nasal discharge scores, each 0=no symptoms to 3=severe in the morning (AM); averaged over previous 7 days.
Mean baseline CSS was 6.2, 5.9, and 6.0, respectively, for EDS-placebo, XHANCE 186 mcg, and XHANCE 372 mcg. At Week 4, CSS was –0.8, –1.5, and –1.7, respectively, for EDS-placebo, XHANCE 186 mcg, and XHANCE 372 mcg. The least squares (LS) mean difference in CSS at Week 4 was –0.7 (95% confidence interval [CI]: –1.3, –0.2) in the
XHANCE 186 mcg group vs EDS-placebo, and –0.9 (95% CI: –1.5, –0.4) in the XHANCE 372 mcg group vs EDS-placebo.1-3
Results shown are from ReOpen2 and are consistent with the results of non-polyp patients seen in ReOpen1 (n=122).1,3
Coprimary endpoint: Sinus opacification in ReOpen2 (without nasal polyps) at Week 241,2
- LS mean change from baseline in percent opacified sinus volume was 0.4 for EDS-placebo, –7.0 (–7.5 [95% CI: –12.1, –2.8] vs placebo) for XHANCE 186 mcg BID, and –5.5 (–5.9 [95% CI: –10.6, –1.3] vs placebo) for XHANCE 372 mcg BID
- Baseline mean percent opacified sinus volume scores were 64.1% for EDS-placebo (n=75), 60.5% for XHANCE 186 mcg BID (n=72), and 61.5% for XHANCE 372 mcg BID (n=73)
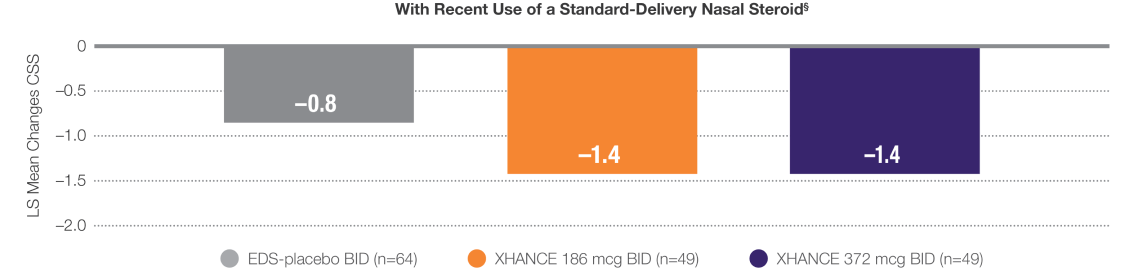
Improvement in symptoms in patients who entered the trial symptomatic despite recent use of standard-delivery nasal steroids
Pooled Data From ReOpen1 and ReOpen2 (Patients Without Nasal Polyps): CSS‡ Change From Baseline to Week 42
LIMITATION: Use of a standard-delivery nasal steroid 30 days prior to study entry was patient-reported and compliance was not confirmed.
‡CSS: Summed congestion, facial pain/pressure, and nasal discharge scores, each 0=no symptoms to 3=severe in the morning (AM); averaged over previous 7 days.3
§Recent use is defined as within 30 days of trial entry or less.2
Improvements in symptoms in patients who entered the trial symptomatic despite prior sinus surgery1,2
Reduction in CSS of congestion/obstruction, facial pain/pressure, and nasal discharge2
Data From ReOpen2 (Patients Without Nasal Polyps)2
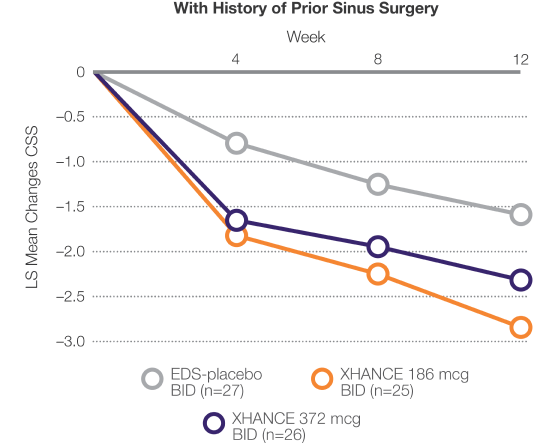

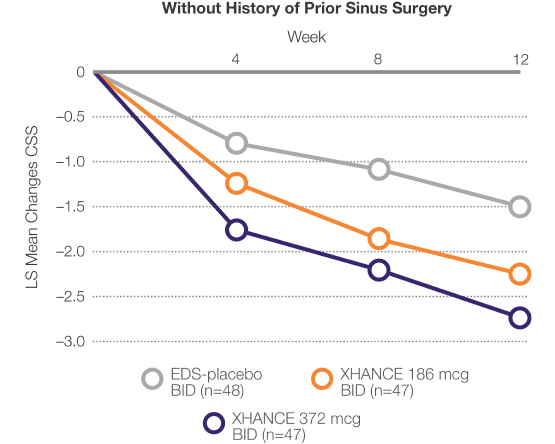

LIMITATION: This pre-planned subgroup analysis was not multiplicity adjusted; results are descriptive and should be interpreted with caution.
More information on XHANCE pivotal trials

Review details of ReOpen1 and ReOpen2 in the Journal of Allergy and Clinical Immunology: In Practice

Review details of NAVIGATE I and NAVIGATE II in the Journal of Allergy and Clinical Immunology
NOTE: These scientific publications contain additional outcome measures and other information, including exploratory endpoints, not contained in the full Prescribing Information, which are subject to important limitations.
In Pooled Data of All Patients From ReOpen1 and ReOpen2
XHANCE showed reductions in acute exacerbations* of chronic sinusitis at 24 weeks1,2
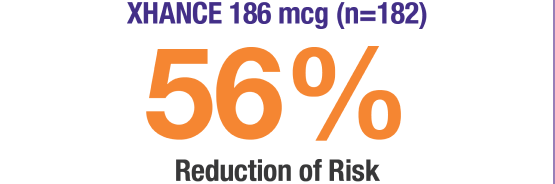

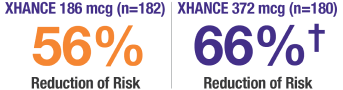
*Data were pooled to assess the incidence of acute exacerbations of chronic rhinosinusitis, defined as a worsening of symptoms that required escalation of treatment (eg, antibiotics, oral steroids, acute care visits). Total number of acute exacerbations of chronic rhinosinusitis (percentage of patients who experienced at least 1 event): EDS-placebo, 41 (15.7%); XHANCE 186 mcg, 20 (9.9%); XHANCE 372 mcg, 15 (7.8%). Incidence rate ratio (IRR) for XHANCE 186 mcg twice daily (BID): 0.44 (95% confidence interval [CI]: 0.2, 0.8) vs EDS-placebo; not controlled for multiplicity.1-3
†IRR for XHANCE 372 mcg BID: 0.34 (95% CI: 0.2, 0.7; P ≤0.05) vs EDS-placebo; type I error-controlled analysis.2
ReOpen1 included 330 patients with either chronic rhinosinusitis without nasal polyps (n=122) or chronic rhinosinusitis with nasal polyps (n=208), and ReOpen2 included 223 patients with chronic rhinosinusitis without nasal polyps.1

Antibiotics were prescribed in 93% of acute exacerbations3

XHANCE is the FIRST and ONLY medication proven to reduce the risk of acute exacerbations of chronic sinusitis1,4
In the subgroup of patients without nasal polyps from ReOpen1 and ReOpen21,2
The rate of acute exacerbations of chronic rhinosinusitis was reduced by 53% among patients in both XHANCE treatment groups vs placebo. This was derived from an IRR of 0.47 (95% CI: 0.2, 1.1) and 0.47 (95% CI: 0.2, 1.1) among patients using XHANCE 186 mcg (n=113) and 372 mcg (n=112) BID vs placebo, respectively; the difference was not statistically significant.
More information on XHANCE pivotal trials

Review details of ReOpen1 and ReOpen2 in the Journal of Allergy and Clinical Immunology: In Practice

Review details of NAVIGATE I and NAVIGATE II in the Journal of Allergy and Clinical Immunology
NOTE: These scientific publications contain additional outcome measures and other information, including exploratory endpoints, not contained in the full Prescribing Information, which are subject to important limitations.
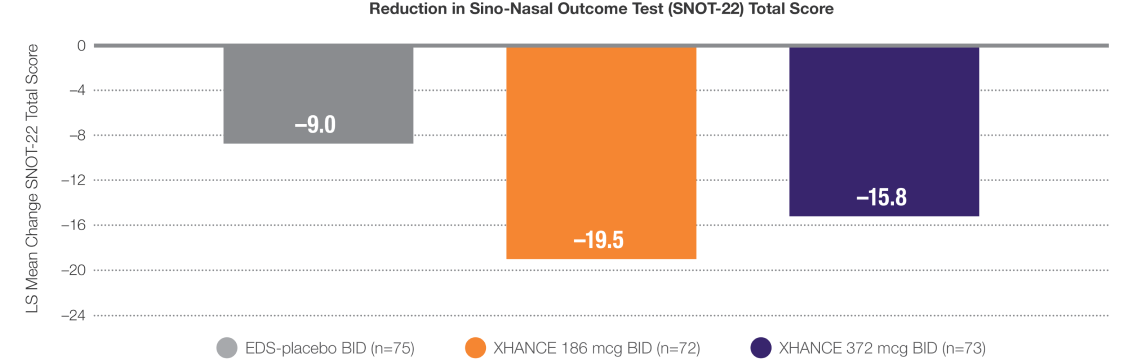
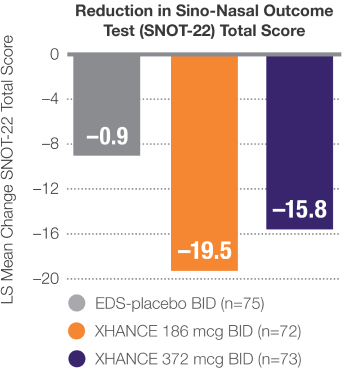
Improvement in SNOT-22 scores1
Data from ReOpen2 (patients without nasal polyps)
LS Mean Change From Baseline at Week 241
BID=twice daily; LS=least squares.
LIMITATION: This pre-planned analysis was not multiplicity adjusted; results are descriptive and should be interpreted with caution.
Surgery has been reported to reduce SNOT-22 scores by 18 to 23 points2,3
- Improvements in SNOT-22 scores were assessed as early as 4 weeks and continued to 24 weeks1
- Minimal clinically important difference of –9 is considered a clinically relevant improvement2,3
More information on XHANCE pivotal trials

Review details of ReOpen1 and ReOpen2 in the Journal of Allergy and Clinical Immunology: In Practice

Review details of NAVIGATE I and NAVIGATE II in the Journal of Allergy and Clinical Immunology
NOTE: These scientific publications contain additional outcome measures and other information, including exploratory endpoints, not contained in the full Prescribing Information, which are subject to important limitations.
NAVIGATE II Study in Adults With Nasal Polyps
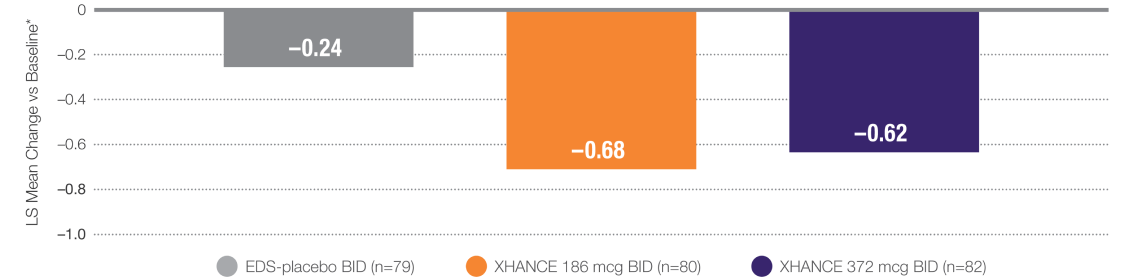
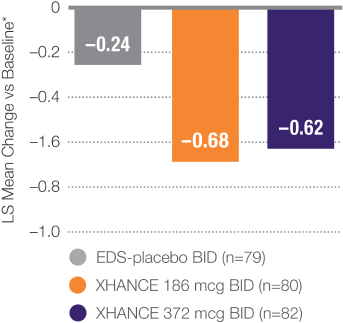
Coprimary Endpoint:
XHANCE Significantly Reduced Congestion/Obstruction in 4 Weeks1,2
P≤0.001 vs EDS-placebo.2
*Least squares (LS) mean change from baseline in patient-reported AM instantaneous daily scores for nasal congestion/obstruction on a scale from 0-3 (0=none, 1=mild, 2=moderate, 3=severe).1
BID=twice daily.
Statistically significant onset of action was generally observed within 2 weeks for congestion/obstruction1
Coprimary Endpoint:
XHANCE Significantly Reduced Polyps in 16 Weeks1,2
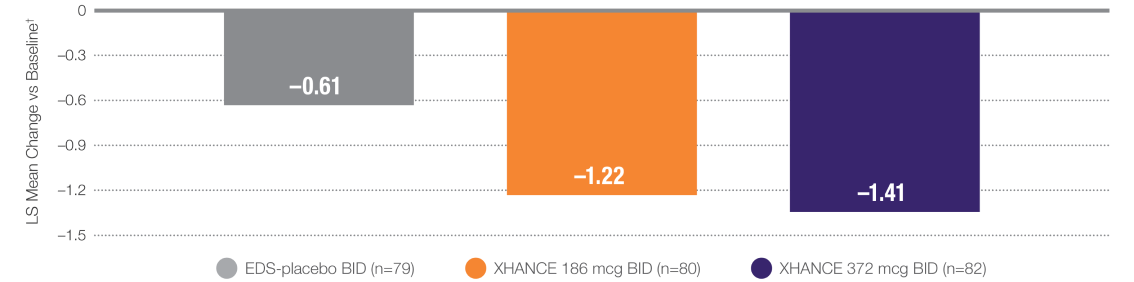
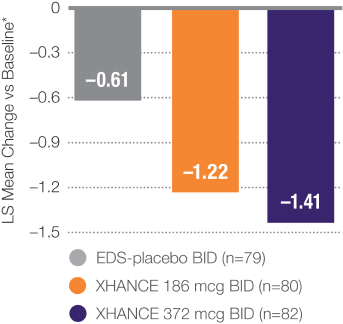
P≤0.001 vs EDS-placebo.2
†Polyp grade was determined by the clinician using nasal endoscopy. Polyps on each side of the nose were graded on a categorical scale (0=No polyps; 1=Mild: polyps not reaching below the inferior border of the middle turbinate; 2=Moderate: polyps reaching below the inferior border of the middle concha, but not the inferior border of the inferior turbinate; 3=Severe: large polyps reaching below the lower inferior border of the inferior turbinate).1
Baseline grade: EDS-placebo, 3.8; XHANCE 186 mcg BID, 3.9; XHANCE 372 mcg BID, 3.9.2
Secondary Endpoint:
XHANCE 372 mcg BID treatment group at Week 162


Continued reduction in bilateral nasal polyp grade during 8-week open label extension following 16-week placebo-controlled trial. All patients received 372 mcg BID during open label extension.2
NOTE: Multiplicity adjustments were not applied for secondary endpoints; therefore, results could potentially represent chance findings.
More information on XHANCE pivotal trials

Review details of ReOpen1 and ReOpen2 in the Journal of Allergy and Clinical Immunology: In Practice

Review details of NAVIGATE I and NAVIGATE II in the Journal of Allergy and Clinical Immunology
NOTE: These scientific publications contain additional outcome measures and other information, including exploratory endpoints, not contained in the full Prescribing Information, which are subject to important limitations.
NAVIGATE I and II Study Design
Two similar randomized, placebo-controlled, multicenter studies to assess XHANCE safety and efficacy in adults with nasal polyps (n=646).1-3
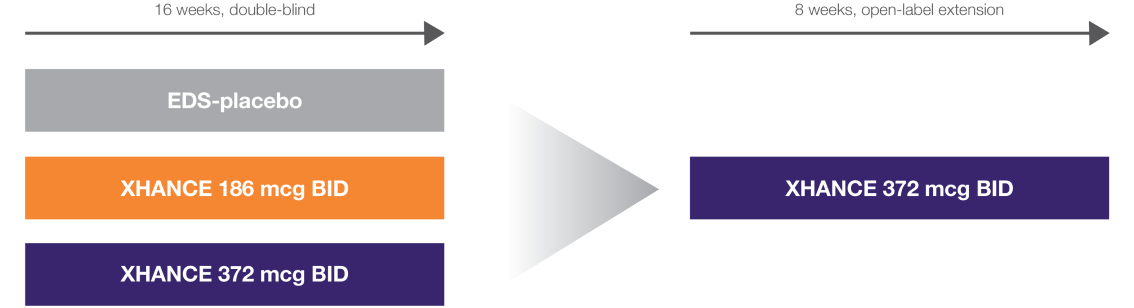
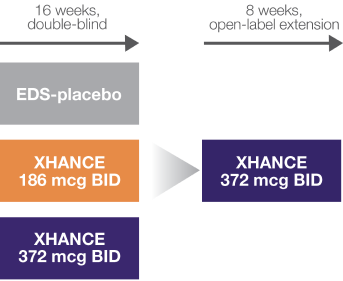
NAVIGATE I: EDS-placebo, n=82; XHANCE 186 mcg BID, n=80; XHANCE 372 mcg BID, n=80. NAVIGATE II: EDS-placebo, n=79; XHANCE 186 mcg BID, n=80; XHANCE 372 mcg BID, n=82.1
Coprimary endpoints1:
- Improvement in nasal congestion/obstruction at Week 4
- Reduction in bilateral polyp grade at Week 16
Secondary endpoints include2,3:
- Change from baseline in sense of smell, rhinorrhea, and facial pain or pressure through Week 12
- Subject assessment of Patient Global Impression of Change (PGIC) at Week 16
- Change in Sino-Nasal Outcome Test-22 (SNOT-22) scores at Week 16
NOTE: Multiplicity adjustments were not applied for secondary endpoints; therefore, results could potentially represent chance findings. Furthermore, open-label results may be confounded by evaluator bias.
Key inclusion criteria1:
- Bilateral nasal polyps (grade 1 to 3)
- Moderate-to-severe symptoms of nasal congestion/obstruction
Details2,3:
- The comparator used in the pivotal clinical studies was a liquid placebo delivered with an Exhalation Delivery SystemTM (EDS®)
- Patients and physicians remained blinded to initial treatment throughout the 8-week open-label extension
- Patients with history of allergic rhinitis could participate in the study provided their “season” did not coincide with the first 4 weeks of the study
- Subjects were allowed to use nonsedating antihistamines as “rescue” after Week 4 in an effort to reduce placebo dropout
Results shown are from NAVIGATE II and are consistent with the results seen in NAVIGATE I2,3
- Statistically significant onset of action was generally observed within 2 weeks1
- Continued improvement of congestion scores through Week 16 of placebo-controlled trial—NAVIGATE II2


- 91% had reported previous use of a nasal steroid for the treatment of nasal polyps
- 54% reported previous sinus surgery or polypectomy
Explore More
Review the safety and tolerability profile
What makes XHANCE different