The Efficacy of XHANCE
- ReOpen1 & ReOpen2
Overview & Study Design - ReOpen1 & ReOpen2
Efficacy Results - ReOpen1 & ReOpen2
Acute Exacerbations - NAVIGATE II Study
Study design of trials
ReOpen1 and ReOpen2 are phase 3, double-blinded trials assessing XHANCE 186 mcg or 372 mcg (1 or 2 sprays/nostril) twice daily (BID) vs EDS-placebo. ReOpen1 (N=332) included patients with or without nasal polyps, whereas ReOpen2 (N=223) excluded patients with nasal polyps. The objective of both was to assess the efficacy of XHANCE for chronic rhinosinusitis (irrespective of nasal polyps).1,2
Polyps extending beyond the attachment of the inferior turbinate
Any observed polyps at all, defined as more than grade 0
(Patient Global Impression of Change [PGIC]) at Week 24
| Select Inclusion Criteria2 | Select Exclusion Criteria2 | ||
|---|---|---|---|
| 18 years or older | Septal perforation | ||
| Diagnosis of chronic rhinosinusitis consistent with guidelines* | Sino-nasal surgery within 6 months | ||
| Confirmatory CT evidence of sinus disease† | ReOpen1 Polyps extending beyond the attachment of the inferior turbinate |
ReOpen2 Any observed polyps at all, defined as more than grade 0 |
|
| Coprimary Endpoints2 | Secondary Endpoints2 | ||
|---|---|---|---|
| Change from baseline at Week 4 in Composite Symptom Score (CSS)‡ | Composite and individual symptom scores over time, through Week 12 | ||
| Change from baseline to Week 24 in sinus opacification1,3§ | Disease-specific quality of life (QoL) (22-item Sino-Nasal Outcome Test [SNOT-22] total score) at Week 24 | ||
| Patient-perceived global change in disease (Patient Global Impression of Change [PGIC]) at Week 24 |
Pooled data endpoints from ReOpen1 and ReOpen22:
- CSS change at Week 4 for patients who were symptomatic (met entry criteria) despite using a standard-delivery nasal steroid at trial entry
- Frequency of acute exacerbations||
†As indicated by 1 or more sinus on each side with Lund-Mackay score of 1 or more, combined ≥25% opacification of the ethmoid sinuses, and ≥25% opacification of at least 1 maxillary sinus.2
‡CSS: Sum of scores averaged as rated by the patient in the morning immediately prior to next dose over 7 days for the above symptoms (0=no symptoms to 3=severe; CSS range 0-9).2
§As measured by the average of the percentages of opacified volumes in ethmoid and maxillary sinuses by CT scan.2
||Acute exacerbations of chronic rhinosinusitis were defined as a worsening of symptoms that required escalation of treatment (e.g., antibiotics, oral steroids, acute care visits).2
In patients without nasal polyps
XHANCE significantly improved symptoms1,4,5
Reduced composite symptom score observed during a randomized, double-blind, placebo-controlled, parallel-group, multicenter study.1
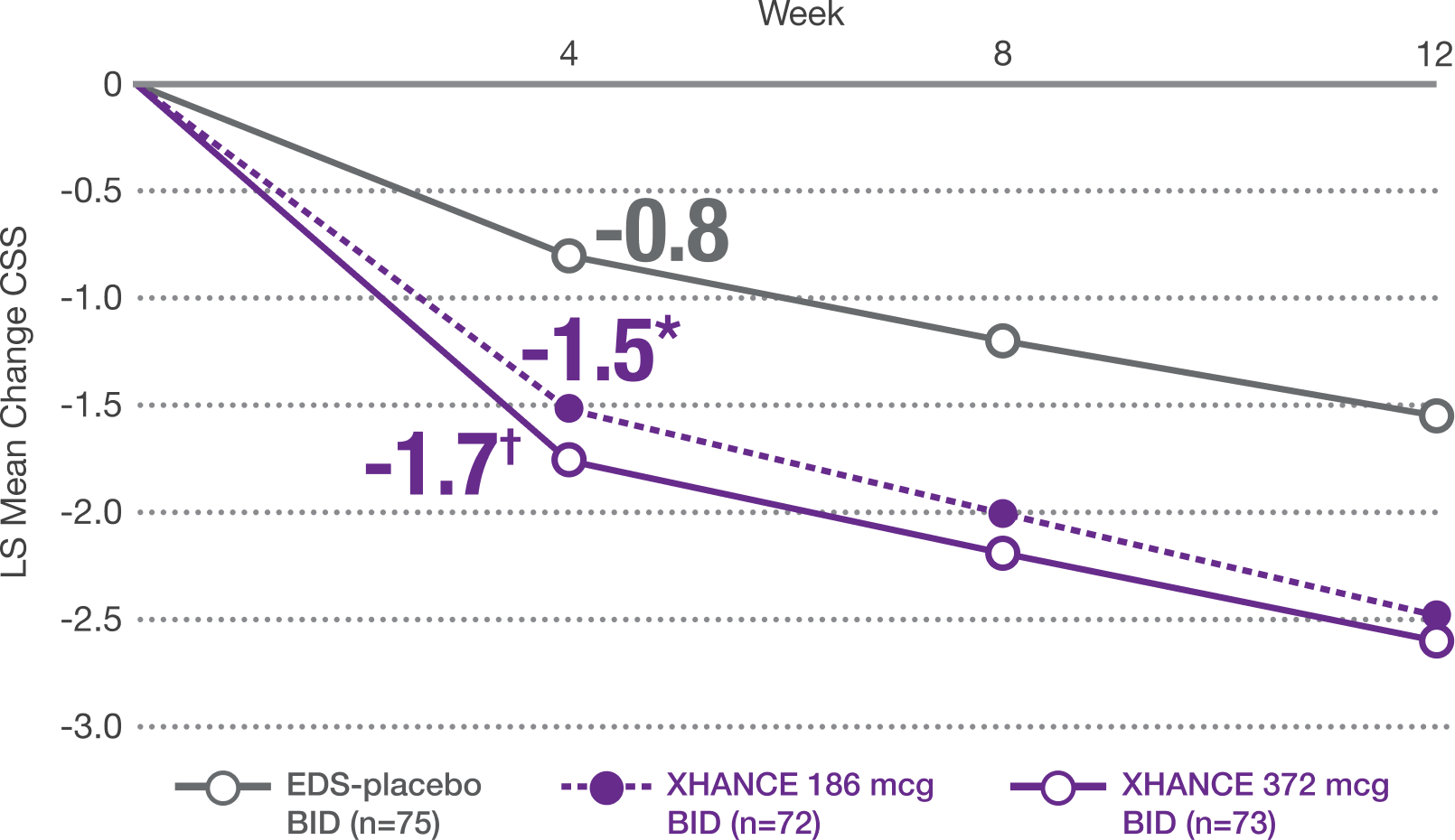
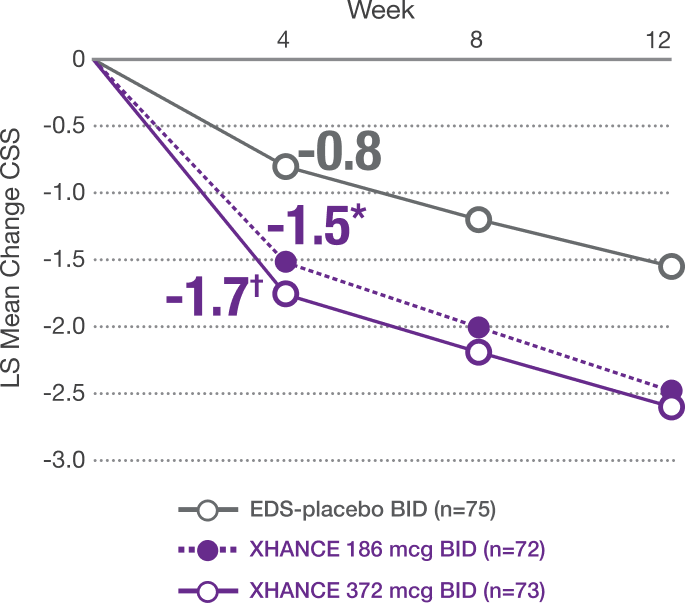
Coprimary Endpoint: Change From Baseline at Week 4 in Composite Symptom Score (CSS) in ReOpen2 (Without Nasal Polyps)1,2
*P≤0.05 vs EDS-placebo. †P≤0.001 vs EDS-placebo.
Results after Week 4 not multiplicity adjusted; results were descriptive and should be interpreted with caution.
CSS: Sum of scores averaged as rated by the patient in the morning immediately prior to next dose over 7 days for the above symptoms (0=no symptoms to 3=severe; CSS range 0-9). Mean baseline CSS was 6.2, 5.9, and 6.0, respectively, for EDS-placebo, XHANCE 186 mcg, and XHANCE 372 mcg. At Week 4, CSS was -0.8, -1.5, and -1.7, respectively, for EDS-placebo, XHANCE 186 mcg, and XHANCE 372 mcg. The LS mean difference in CSS at Week 4 was -0.7 (95% CI: -1.3, -0.2) in the XHANCE 186 mcg group vs EDS-placebo, and -0.9 (95% CI: -1.5, -0.4) in the XHANCE 372 mcg group vs EDS-placebo.1,2
Study design summary: Patients randomized 1:1:1 to receive XHANCE 186 mcg BID, XHANCE 372 mcg BID or placebo, nasally for 24 weeks. Patients had at least 2 active nasal symptoms with a minimum nasal congestion score ≥1.5 out of 3 and a baseline CT scan showing ≥25% opacification of both ethmoid and at least 1 maxillary sinus.1
Coprimary endpoint: Sinus opacification in ReOpen2 (without nasal polyps) at Week 24
- LS mean change from baseline in percent opacified sinus volume was 0.4 for EDS-placebo, -7.0 (-7.5 [95% CI: -12.1, -2.8] vs EDS-placebo) for XHANCE 186 mcg BID, and -5.5 (-5.9 [95% CI: -10.6, -1.3] vs EDS-placebo) for XHANCE 372 mcg BID1
- Baseline mean percent opacified sinus volume scores were 64.1% for EDS-placebo (n=75), 60.5% for XHANCE 186 mcg BID (n=72), and 61.5% for XHANCE 372 mcg BID (n=73)1
BID=twice daily; CI=confidence interval; CSS=Composite Symptom Score; EDS=Exhalation Delivery System; LS=least squares.
In patients without nasal polyps
Improvement in symptomatic patients who recently used standard-delivery nasal steroids5
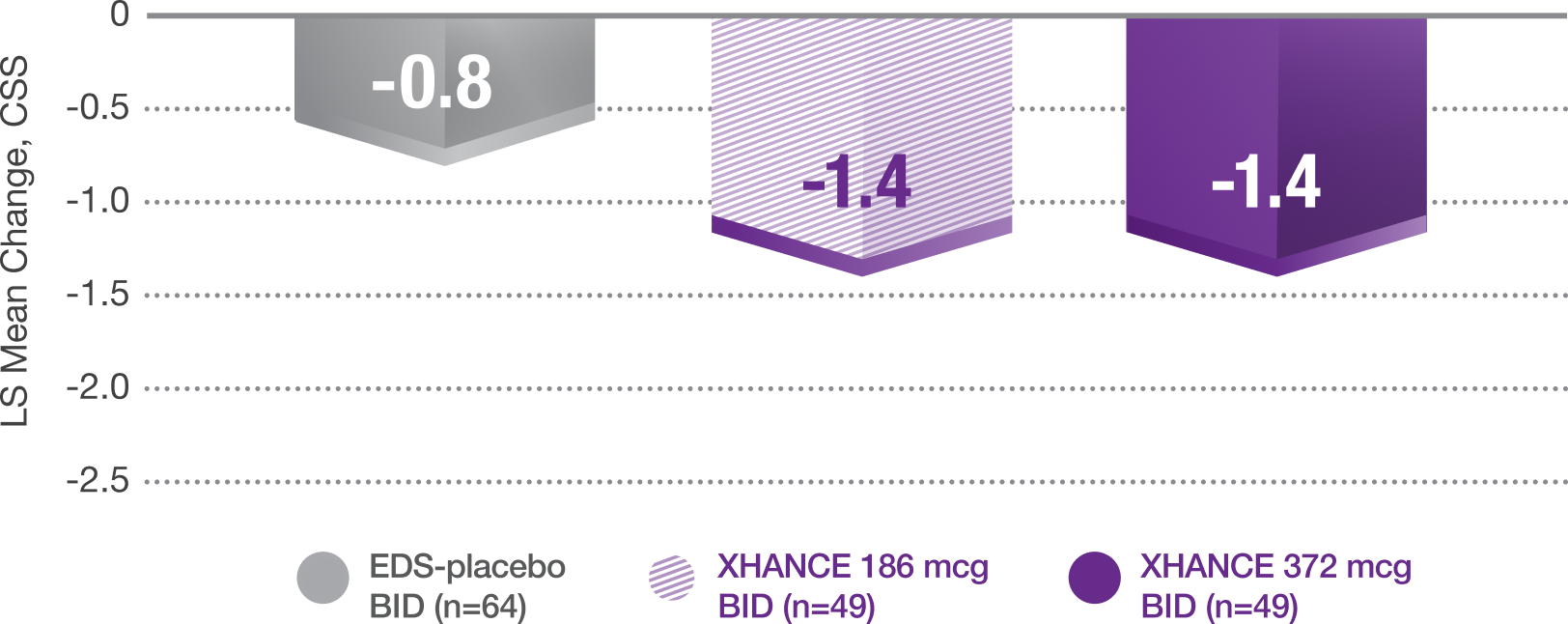
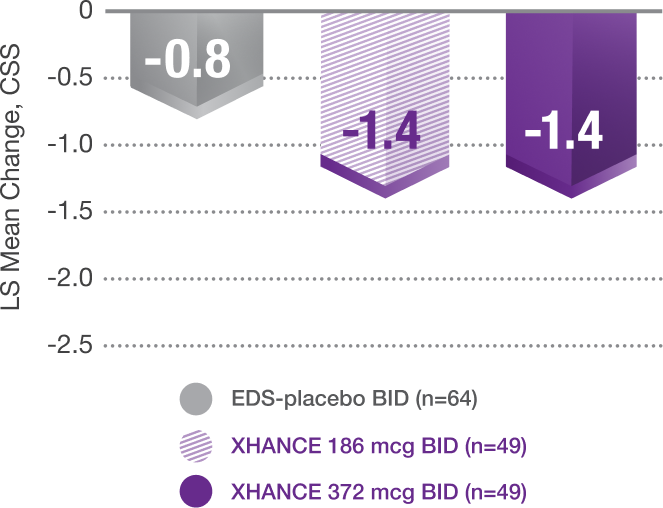
Pooled data from ReOpen1 and ReOpen2 (Patients without nasal polyps):
CSS* change from baseline at Week 45
With Recent Use of a Standard-Delivery Steroid†
CSS LIMITATION: Use of a standard-delivery nasal steroid 30 days prior to study entry was patient-reported and compliance was not confirmed.
*Core symptoms of chronic sinusitis are defined as congestion/obstruction, facial pain/pressure, and nasal discharge.1
†Using at least one standard nasal spray at trial entry.1,4
BID=twice daily; CSS=Composite Symptom Score; EDS=Exhalation Delivery System; LS=least squares.
This analysis was not adjusted for multiplicity; results are descriptive and should be interpreted with caution.
Pooled data from 2 trials of patients with or without nasal polyps
Acute exacerbations* were reduced at 24 weeks5
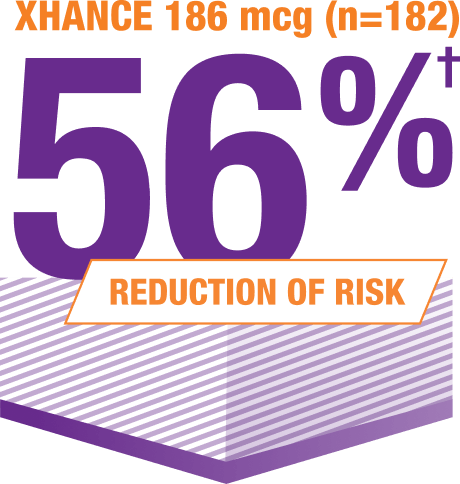
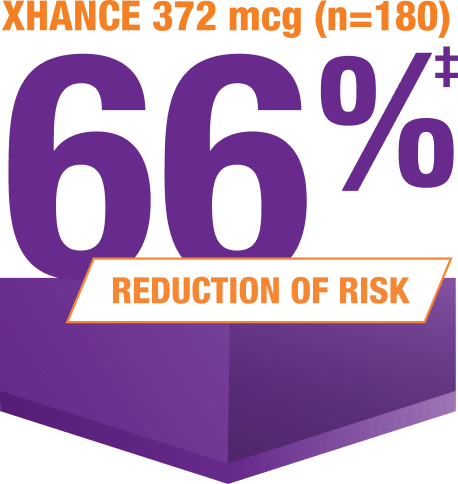
*Data were pooled to assess the incidence of acute exacerbations of CRS, defined as a worsening of symptoms that required escalation of treatment (eg, antibiotics, oral steroids, acute care visits).1,2,5
†IRR for XHANCE 186 mcg BID: 0.44; (95% CI 0.23); not controlled for multiplicity.2
‡IRR for XHANCE 372 mcg BID: 0.34 (95% CI: 0.2, 0.7; P=0.002) vs EDS-placebo; type I error-controlled analysis.5
ReOpen1 included 332 patients with CRS with or without nasal polyps and ReOpen2 included 223 patients with CRS without nasal polyps.1
XHANCE is the first and only medication shown to reduce the risk of occurrence of acute exacerbations of CRS without polyps1,6,7
In the subgroup of patients without nasal polyps from ReOpen1 and ReOpen2, the rate of acute exacerbations of CRS was reduced by 53% among patients in both XHANCE treatment groups vs placebo. This was derived from an IRR of 0.47 (95% CI: 0.2, 1.1) and 0.47 (95% CI: 0.2, 1.1) among patients using XHANCE 186 mcg (n=113) and 372 mcg (n=112) twice daily vs placebo, respectively; the difference was not statistically significant.1
IRR=incidence rate ratio.
In patients with nasal polyps
Congestion/obstruction was significantly reduced at Week 41,8
Coprimary Endpoint: Change From Baseline at Week 4 in Nasal Congestion in NAVIGATE II (Patients With Nasal Polyps)
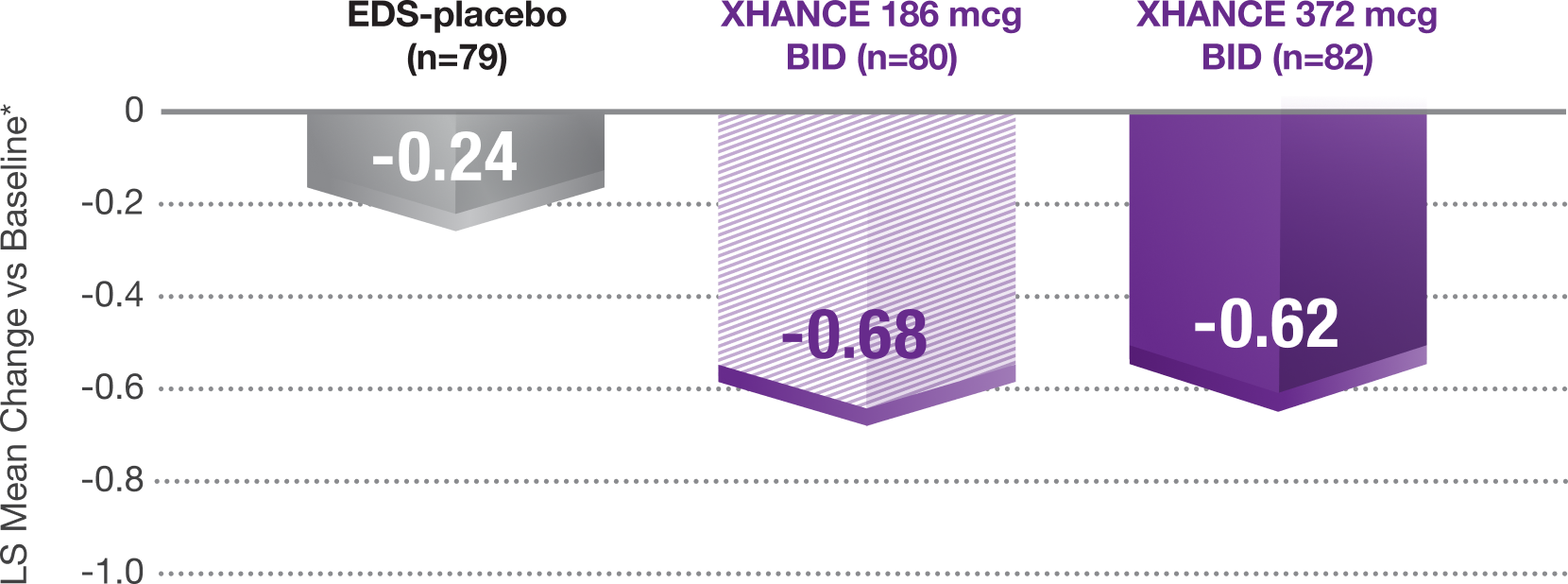
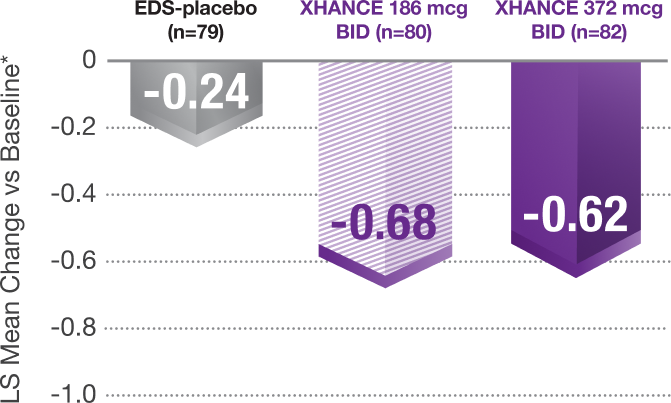
*Least-squares mean change from baseline in patient-reported AM instantaneous daily scores for nasal symptoms on a scale from 0-3 (0=none, 1=mild, 2=moderate, 3=severe).1
The majority of patients in the NAVIGATE II trial had ≥1 polyp grade reduction at Week 16 with both XHANCE 186 mcg (n=80) and 372 mcg (n=82) BID doses. XHANCE 186 BID: 63.0% (OR 2.32); XHANCE 372 mcg BID: 69.1% (OR 3.09).8
P<0.001 vs EDS-placebo.
Trial results
- Onset of action was generally observed within 2 weeks1,8
- Continued improvement of congestion scores through week 16 of placebo-controlled trial—NAVIGATE II8
- Statistically significant reduction in bilateral polyp grade at Week 161
Study design summary: 16-week, randomized, double-blind, parallel-group, multicenter, placebo-controlled trials in adults ≥18 years with CRS with nasal polyps and moderate-to-severe nasal congestion. Patients received 186 mcg or 372 mcg XHANCE BID or placebo BID. Coprimary efficacy endpoints: 1) Change from baseline to Week 4 in nasal congestion/obstruction averaged over preceding 7 days of treatment and 2) change from baseline to Week 16 in bilateral polyp grade. Congestion rated by patient on 4-point severity scale (0=none, 1=mild, 2=moderate, 3=severe) immediately prior to next dose. Polyps determined by nasal endoscopy: 0=No polyps; 1=Mild: polyps not reaching below inferior border of middle turbinate; 2=Moderate: polyps reaching below inferior border of middle concha, but not inferior border of inferior turbinate; 3=Severe: large polyps reaching below lower inferior border of inferior turbinate.1
BID=twice daily; OR=odds ratio.
Explore More
Review the safety and tolerability profile
Explore the XHANCE delivery system