The Efficacy of XHANCE
![]()
Coprimary endpoint:
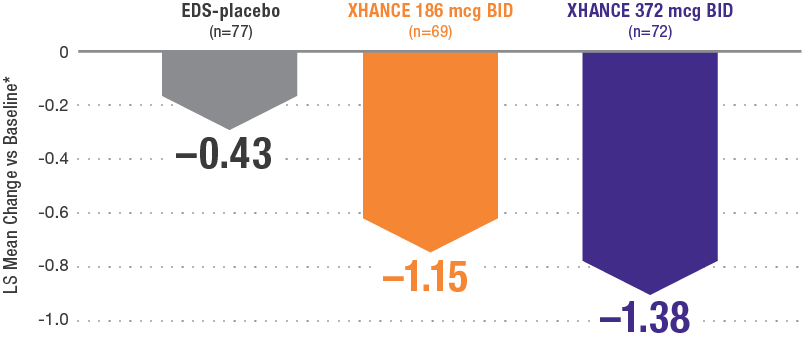
XHANCE significantly reduced congestion/obstruction in 4 weeks1,2
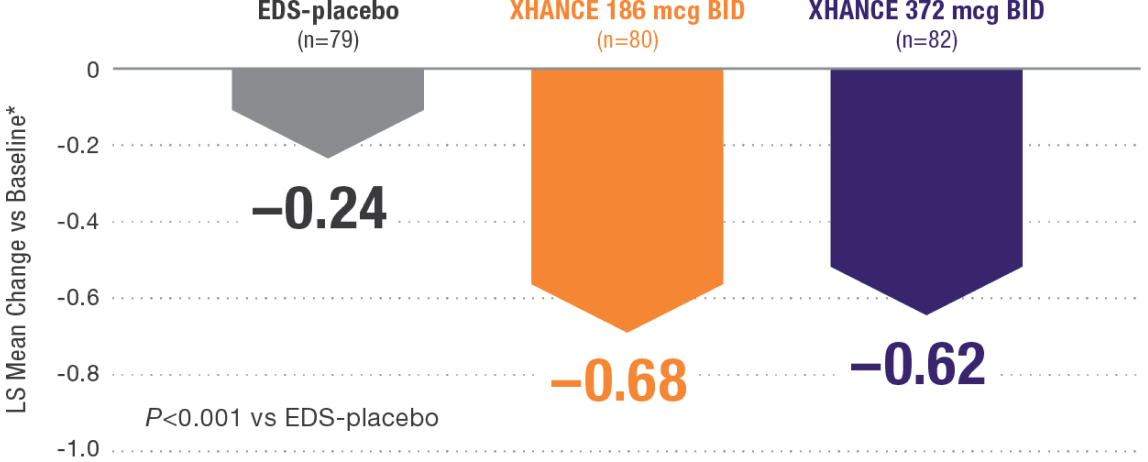
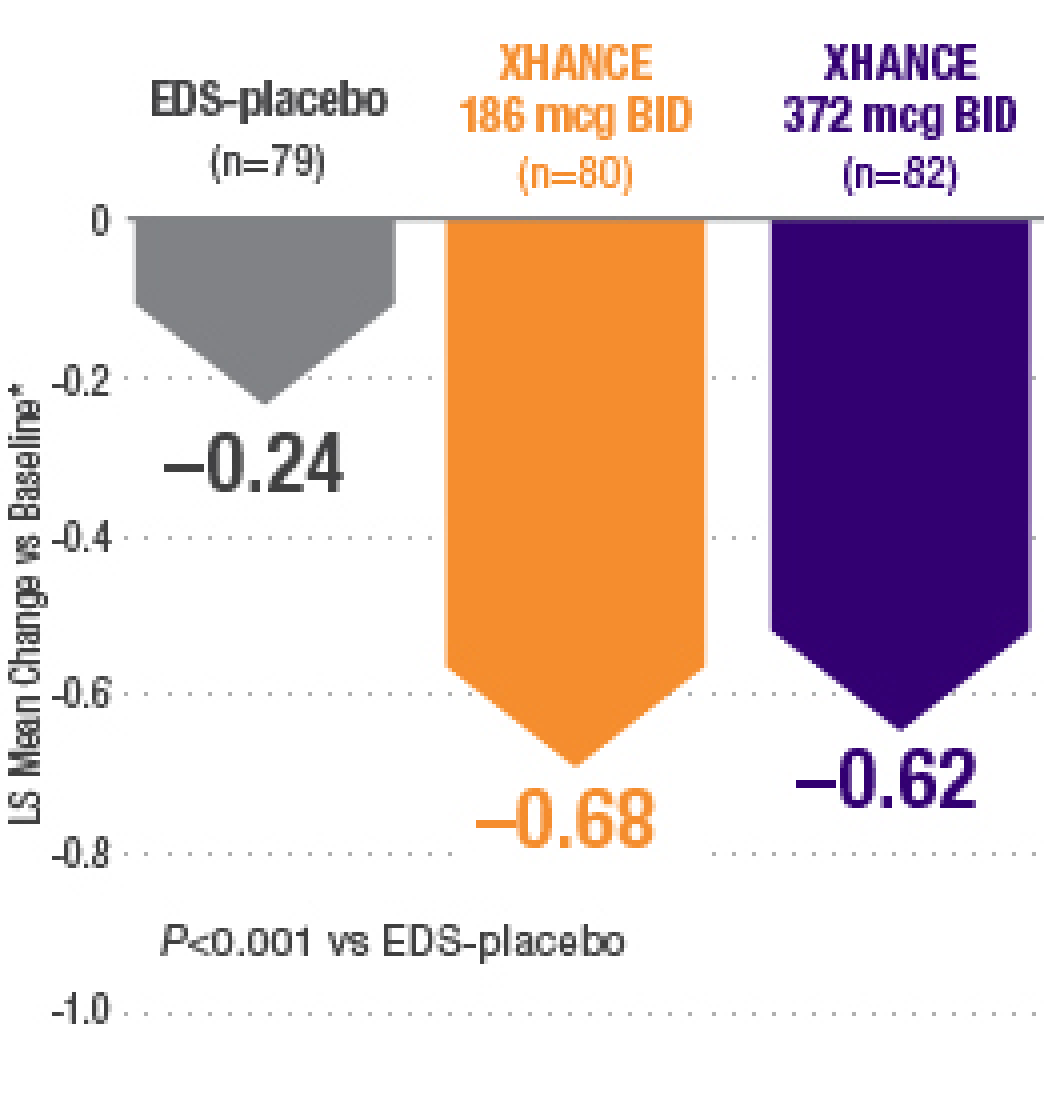
*Least-squares mean change from baseline in patient-reported AM instantaneous daily scores for nasal symptoms on a scale from 0-3 (0=none, 1=mild, 2=moderate, 3=severe).1 BID=twice daily.
Results shown are from NAVIGATE II and are consistent with the results seen in NAVIGATE I.2,3
- Statistically significant onset of action was generally observed within 2 weeks1
- Continued improvement of congestion scores through week 16 of placebo-controlled trial—NAVIGATE II2
NAVIGATE I and NAVIGATE II were double-blind, placebo-controlled trials lasting 16 weeks with an 8-week open-label extension in patients with bilateral nasal polyps and moderate to severe congestion. All patients received XHANCE 372 mcg BID during the open-label extension.1-3
XHANCE trial design & details
XHANCE is a corticosteroid indicated for the treatment of nasal polyps in patients 18 years of age or older.XHANCE has been extensively studied in over 1500 patients.1-5
646 patients with bilateral nasal polyps evaluated for efficacy and safety for up to 24 weeks2,3
- NAVIGATE I and NAVIGATE II were double-blind, placebo-controlled trials lasting 16 weeks with an 8-week open-label extension in patients with bilateral nasal polyps and moderate to severe congestion. All patients received XHANCE 372 mcg BID during the open-label extension1-3
928 patients with chronic sinusitis with and without nasal polyps in 2 open-label trials evaluated for safety for up to 12 months1,4
- EXHANCE-3 and EXHANCE-12 were open-label studies lasting 3 months (n=705) and 12 months (n=223)1,4

In NAVIGATE I and NAVIGATE II, XHANCE was studied in a patient population in which1:
- 91% had reported previous use of a nasal steroid for the treatment of nasal polyps
- 54% reported previous sinus surgery or polypectomy
NAVIGATE I and NAVIGATE II: Phase 3 trials evaluating efficacy and safety
Two similar randomized, placebo-controlled, multicenter studies to assess XHANCE safety and efficacy (n=646)1


NAVIGATE I: EDS-placebo, n=82; XHANCE 186 mcg BID, n=80; XHANCE 372 mcg BID, n=80. NAVIGATE II: EDS-placebo, n=79; XHANCE 186 mcg BID, n=80; XHANCE 372 mcg BID, n=82.1
Coprimary endpoints1:
- Improvement in nasal congestion/obstruction at Week 4
- Reduction in bilateral polyp grade at Week 16
Secondary endpoints include2,3:
- Change from baseline in sense of smell, rhinorrhea, and facial pain or pressure
- Subject assessment of Patient Global Impression of Change (PGIC) at Week 16
- Change in Sino-Nasal Outcome Test-22 (SNOT-22) scores at Week 16
Key inclusion criteria1:
- Bilateral nasal polyps (grade 1 to 3)
- Moderate-to-severe symptoms of nasal congestion/obstruction
Details2,3,5:
- The comparator used in the pivotal clinical studies was a liquid placebo delivered with an Optinose Exhalation Delivery System (EDS)
- Patients and physicians remained blinded to initial treatment throughout the 8-week open-label extension
- Patients with history of allergic rhinitis could participate in the study provided their “season” did not coincide with the first 4 weeks of the study
- Subjects were allowed to use nonsedating antihistamines as “rescue” after Week 4 in an effort to reduce placebo dropout
EXHANCE-3 and EXHANCE-12: Phase 3 open-label studies
Data demonstrating safety for up to 1 year were also evaluated in 2 open-label studies in 928 patients with chronic sinusitis (CS) with or without nasal polyps.1,4*


Key inclusion criteria4:
- CS with or without nasal polyps*
- Experiencing ≥2 defining symptoms of CS
*XHANCE is not indicated to treat CS.
- Full Prescribing Information for XHANCE (fluticasone propionate). OptiNose US, Inc.; 2023.
- Leopold DA, Elkayam D, Messina JC, et al. NAVIGATE II: randomized, double-blind trial of the exhalation delivery system with fluticasone (EDS-FLU) for nasal polyposis. J Allergy Clin Immunol. 2019;143(1):126-134.
- Sindwani R, Han JK, Soteres DF, et al. NAVIGATE I: randomized, placebo-controlled, double-blind trial of the Exhalation Delivery System with fluticasone for chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2019;33(1):69-82.
- Palmer JN, Jacobson KW, Messina JC, et al. EXHANCE-12: 1-year study of the exhalation delivery system with fluticasone (EDS-FLU) in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2018;8(8):869-876.
- Data on file. OptiNose US, Inc.
CONGESTION/OBSTRUCTION REDUCTION IN PATIENTS PREVIOUSLY USING STANDARD NASAL STEROIDS1,2
Reductions in congestion/obstruction at Week 4 in the subgroup of patients reporting standard nasal steroid use prior to study entry1,2
In a subgroup of patients reporting use of a standard nasal steroid within 30 days of the screening visit1:
- Patients reported a mean treatment duration of 3 years with a standard nasal steroid
- The improvements in this subgroup were similar in magnitude to improvements in overall study population
Post hoc analysis of NAVIGATE I and II
Pooled subgroup data (n=218)
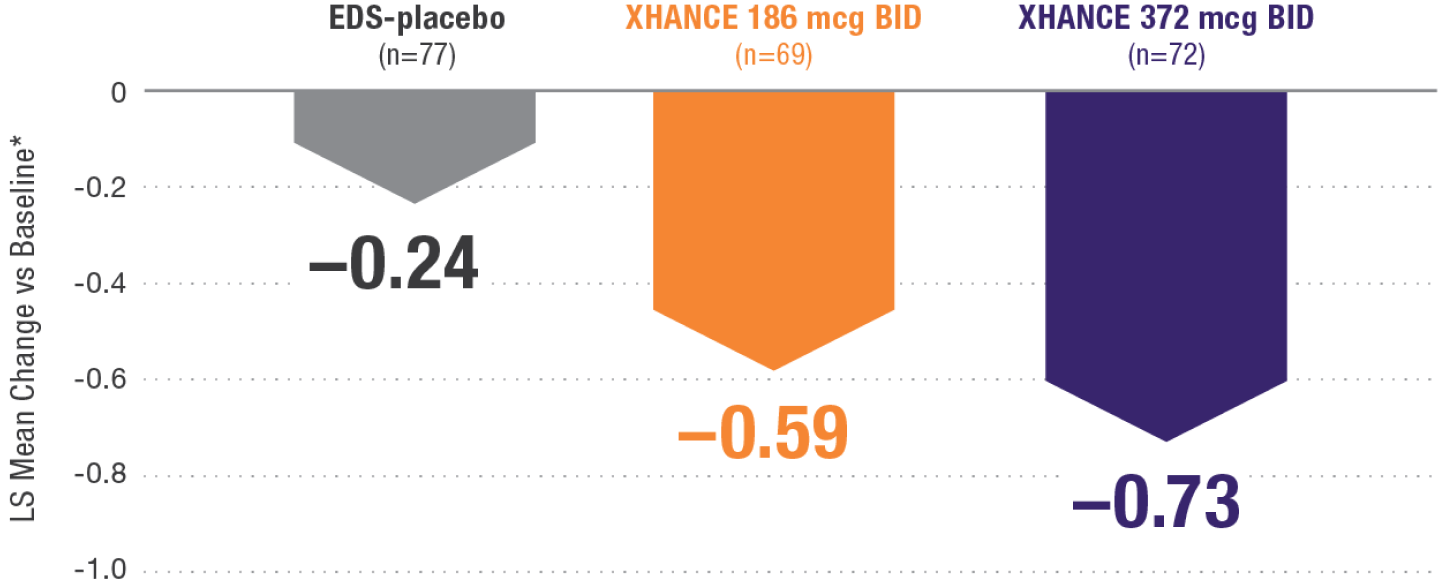
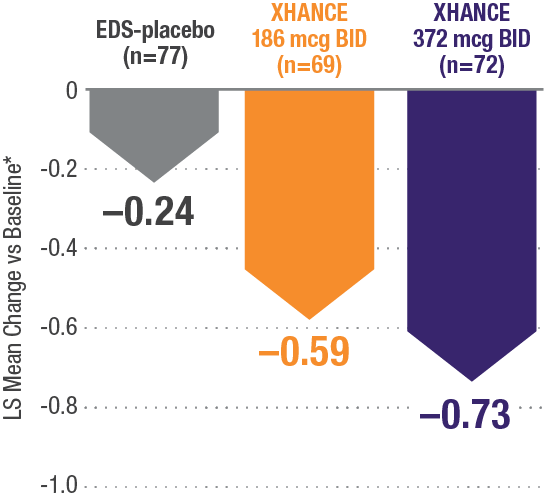
*Least-squares mean change from baseline in patient-reported AM instantaneous daily scores for nasal symptoms on a scale from 0-3 (0=none, 1=mild, 2=moderate, 3=severe).1 BID=twice daily.
- Local Nasal Effects: epistaxis, erosion, ulceration, septal perforation, Candida albicans infection, and impaired wound healing. Monitor patients periodically for signs of possible changes on the nasal mucosa. Avoid use in patients with recent nasal ulcerations, nasal surgery, or nasal trauma until healing has occurred.
- Senior BA, Schlosser RJ, Bosso J, Soler ZM. Efficacy of the exhalation delivery system with fluticasone in patients who remain symptomatic on standard nasal steroid sprays. Int Forum Allergy Rhinol. 2021;11(5):837-845.
- Full Prescribing Information for XHANCE (fluticasone propionate). OptiNose US, Inc.; 2023.

Statistically significant onset of action was generally observed within 2 weeks for congestion/obstruction.1
![]()
Coprimary endpoint:
XHANCE significantly reduced polyps in 16 weeks1,2
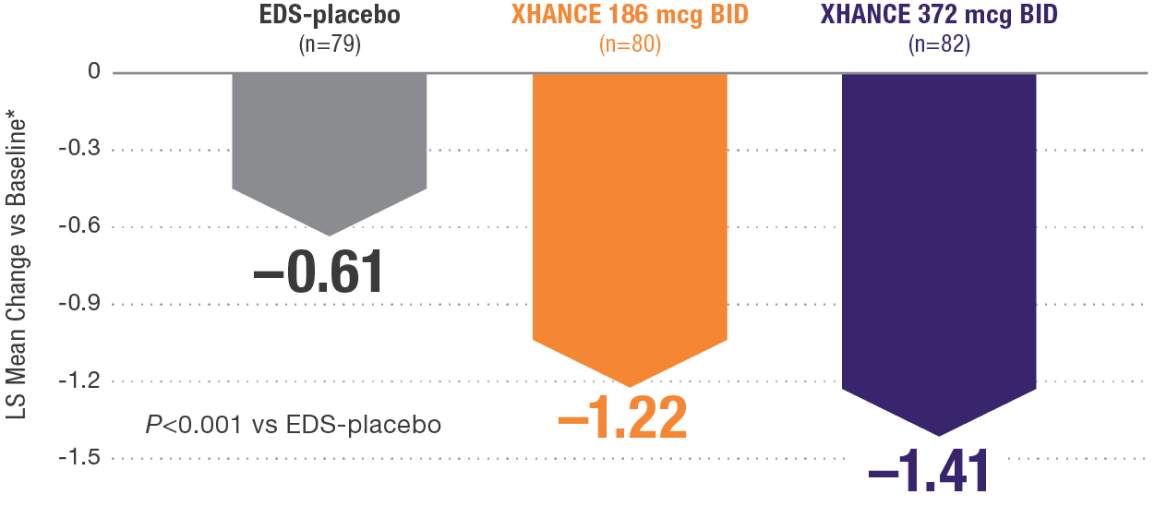
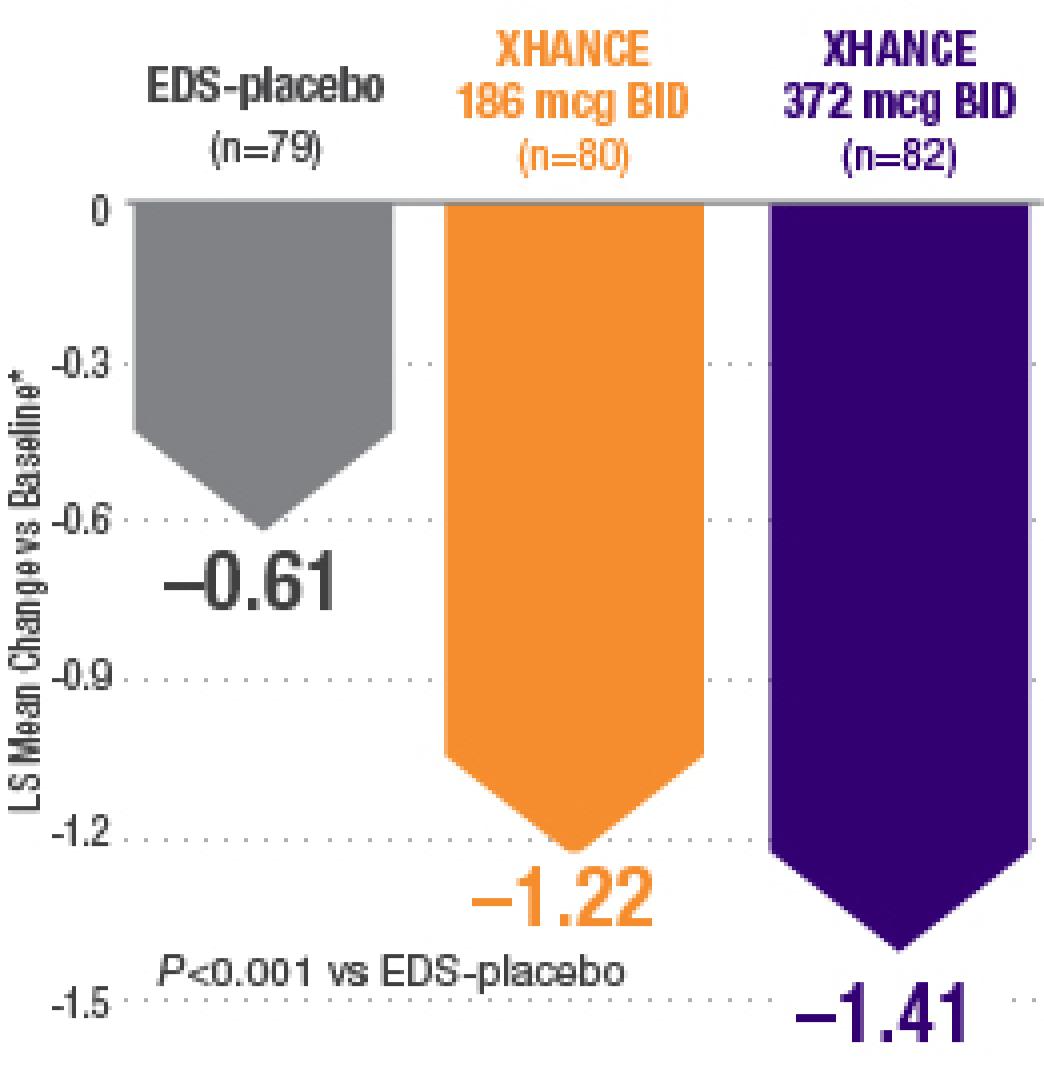
Baseline grade: EDS-placebo, 3.8; XHANCE 186 mcg BID, 3.9; XHANCE 372 mcg BID, 3.9.2
*Polyp grade was determined by the clinician using nasal endoscopy. Polyps on each side of the nose were graded on a categorical scale (0=No polyps; 1=Mild: polyps not reaching below the inferior border of the middle turbinate; 2=Moderate: polyps reaching below the inferior border of the middle concha, but not the inferior border of the inferior turbinate; 3=Severe: large polyps reaching below the lower inferior border of the inferior turbinate).1</sup
Secondary endpoint:
XHANCE 372 mcg BID treatment group at Week 162
Secondary endpoint:
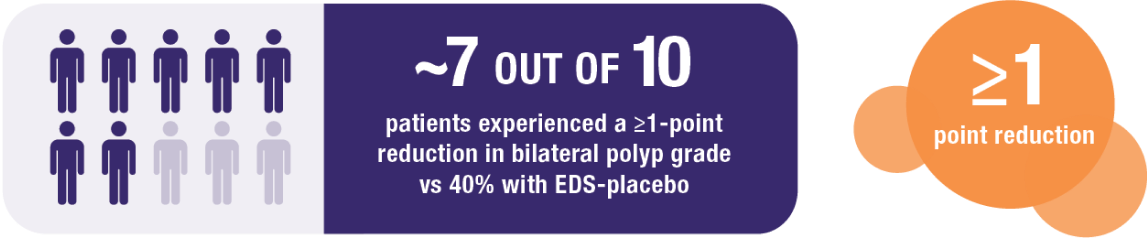
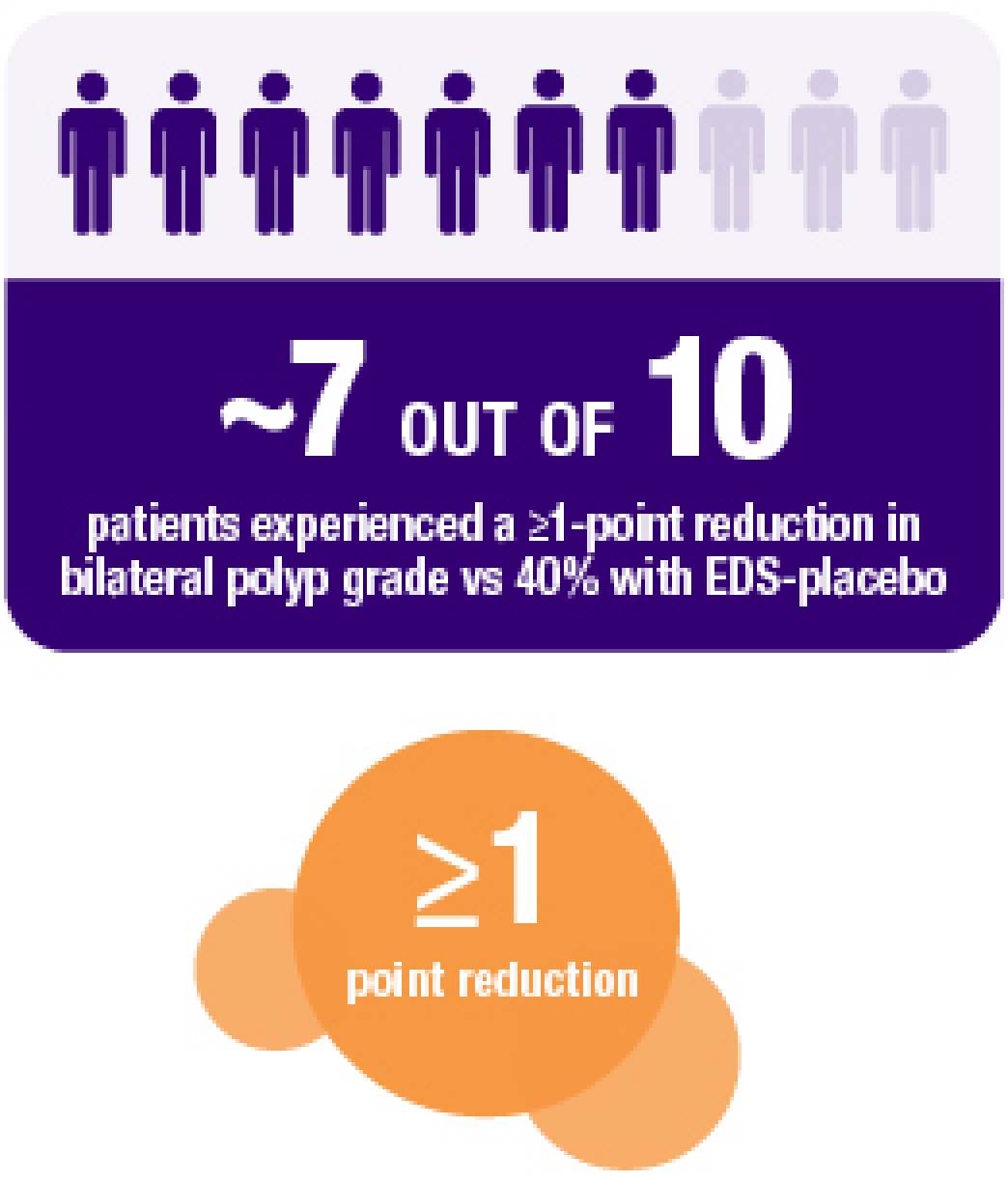
NOTE: Multiplicity adjustments were not applied for secondary endpoints; therefore, results could potentially represent chance findings.
Continued reduction in bilateral nasal polyp grade during 8-week open label extension following 16-week placebo-controlled trial. All patients received 372 mcg BID during open label extension.2
POLYP REDUCTION IN PATIENTS WITH AND WITHOUT PRIOR SINUS SURGERY1
Reduction in bilateral nasal polyp score at Week 16 in the subgroups of patients with or without prior sinus surgery1
- A post hoc analysis of a pooled population from NAVIGATE I and NAVIGATE II analyzed reduction in bilateral polyp grade in the subgroup of patients who had sinus surgery and those who did not have sinus surgery prior to study entry1
- Patients were excluded from the studies if they had a history of sinus or nasal surgery within 6 months before screening2,3
Post hoc analysis of NAVIGATE I and II
Pooled data (n=481)
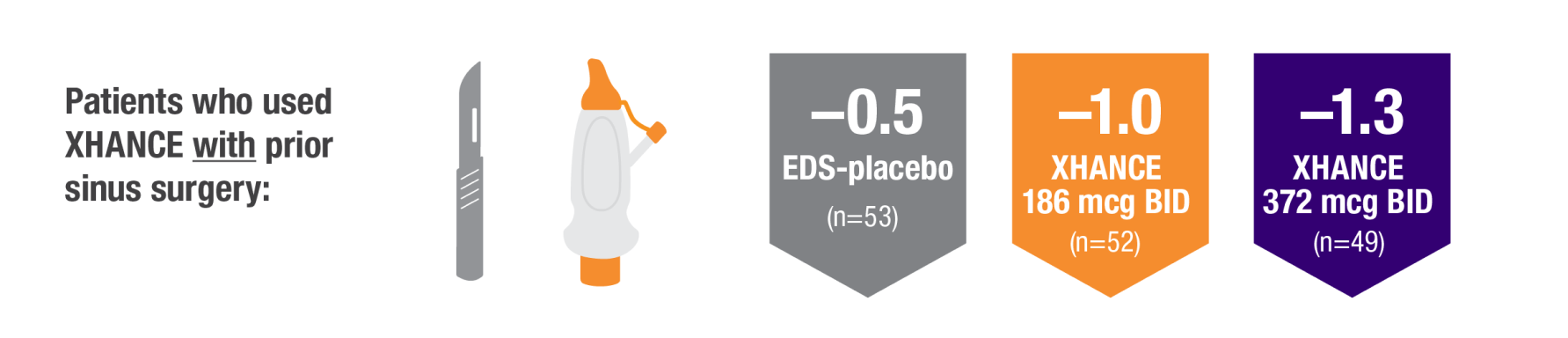
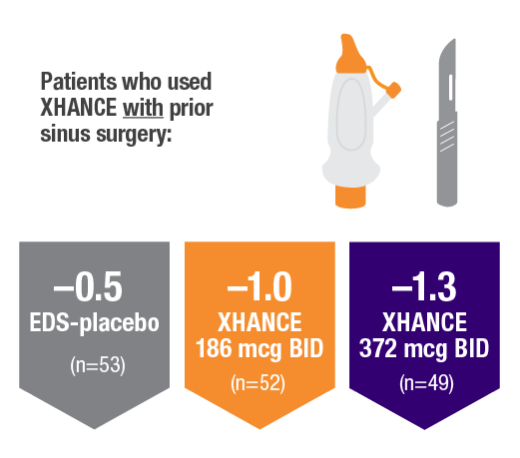
Baseline grade: EDS-placebo, 3.9; XHANCE 186 mcg BID, 4.0; XHANCE 372 mcg BID, 3.9.1-3

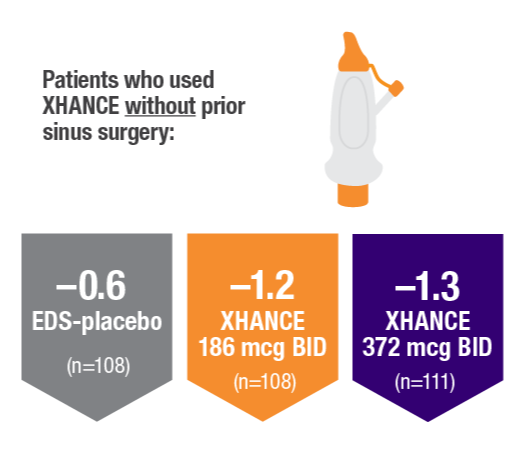
Baseline grade: EDS-placebo, 3.7; XHANCE 186 mcg BID, 3.8; XHANCE 372 mcg BID, 3.7.1
- Local Nasal Effects: epistaxis, erosion, ulceration, septal perforation, Candida albicans infection, and impaired wound healing. Monitor patients periodically for signs of possible changes on the nasal mucosa. Avoid use in patients with recent nasal ulcerations, nasal surgery, or nasal trauma until healing has occurred.
Please see additional Important Safety Information and full Prescribing Information, including Instructions for Use.
- Data on file. OptiNose US, Inc.
- Leopold DA, Elkayam D, Messina JC, et al. NAVIGATE II: randomized double-blind trial of the exhalation delivery system with fluticasone (EDS-FLU) for nasal polyposis. J Allergy Clin Immunol. 2019;143(1):126-134.
- Sindwani R, Han JK, Soteres DF, et al. NAVIGATE I: randomized, placebo-controlled, double-blind trial of the Exhalation Delivery System with fluticasone for chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2019;33(1):69-82.
POLYP REDUCTION IN PATIENTS PREVIOUSLY USING STANDARD NASAL STEROIDS1
Reductions in nasal polyp grade at Week 16 in the subgroup of patients reporting standard nasal steroid use prior to study entry1
In a subgroup of patients reporting use of a standard nasal steroid within 30 days of the screening visit1:
- Patients reported a mean treatment duration of 3 years with a standard nasal steroid
- The improvements in this subgroup were similar in magnitude to improvements in overall study population
Post hoc analysis of NAVIGATE I and II
Pooled subgroup data (n=218)
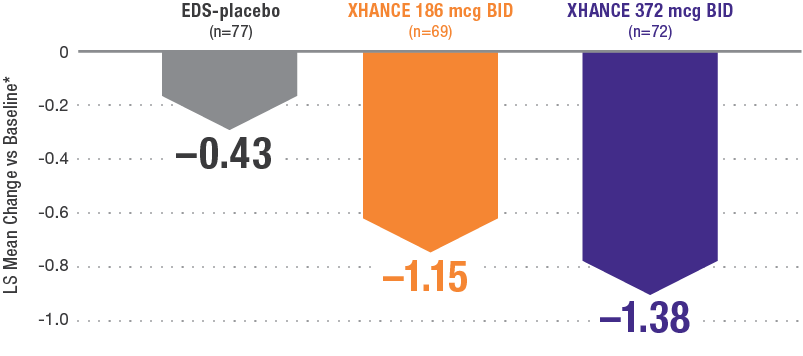
*Polyp grade was determined by the clinician using nasal endoscopy. Polyps on each side of the nose were graded on a categorical scale (0=No polyps; 1=Mild: polyps not reached below the inferior border of the middle turbinate; 2=Moderate: polyps reaching below the inferior border of the middle concha, but not the inferior border of the inferior turbinate; 3=Severe: large polyps reaching below the lower inferior border of the inferior turbinate1
- Local Nasal Effects: epistaxis, erosion, ulceration, septal perforation, Candida albicans infection, and impaired wound healing. Monitor patients periodically for signs of possible changes on the nasal mucosa. Avoid use in patients with recent nasal ulcerations, nasal surgery, or nasal trauma until healing has occurred.
Please see additional Important Safety Information and full Prescribing Information, including Instructions for Use.
- Senior BA, Schlosser RJ, Bosso, J, Soler ZM. Efficacy of the exhalation delivery system with fluticasone in patients who remain symptomatic on standard nasal steroid sprays. Int Forum Allergy Rhinol. 2020.
POLYP GRADE CRITERIA
How polyps were graded in NAVIGATE I and NAVIGATE II
The bilateral polyp grade scale ranges from 0 (no polyp) to 6 (severe bilateral nasal polyposis). Baseline grade in NAVIGATE II: EDS-placebo, 3.8; XHANCE 186 mcg BID, 3.9; XHANCE 372 mcg BID, 3.9.1
- 0=no polyps
- 1=not below the inferior border of the middle turbinate
- 2=below the inferior border of the middle turbinate but not the inferior border of the inferior turbinate
- 3=below the lower inferior border of the inferior turbinate
- Leopold DA, Elkayam D, Messina JC, et al. NAVIGATE II: randomized, double-blind trial of the exhalation delivery system with fluticasone (EDS-FLU) for nasal polyposis. J Allergy Clin Immunol. 2019;143(1):126-134.
- Sindwani R, Han JK, Soteres DF, et al. NAVIGATE I: randomized, placebo-controlled, double-blind trial of the Exhalation Delivery System with fluticasone for chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2019;33(1):69-82.
- Data on file. OptiNose US, Inc.
![]()
Improvement in all 4 defining symptoms of nasal polyps1,4,5
Symptom improvement was observed irrespective of prior corticosteroid and/or surgical treatment.1,4
Coprimary endpoint:

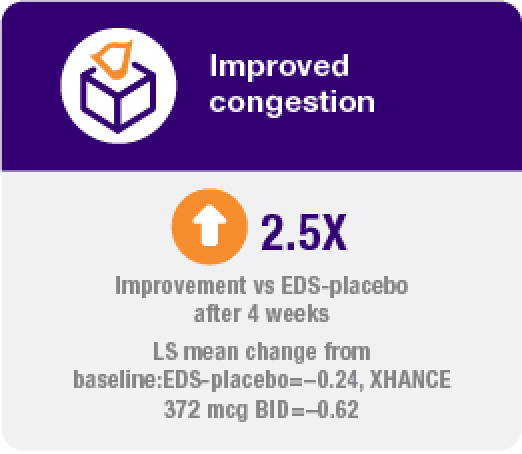
Secondary endpoints:
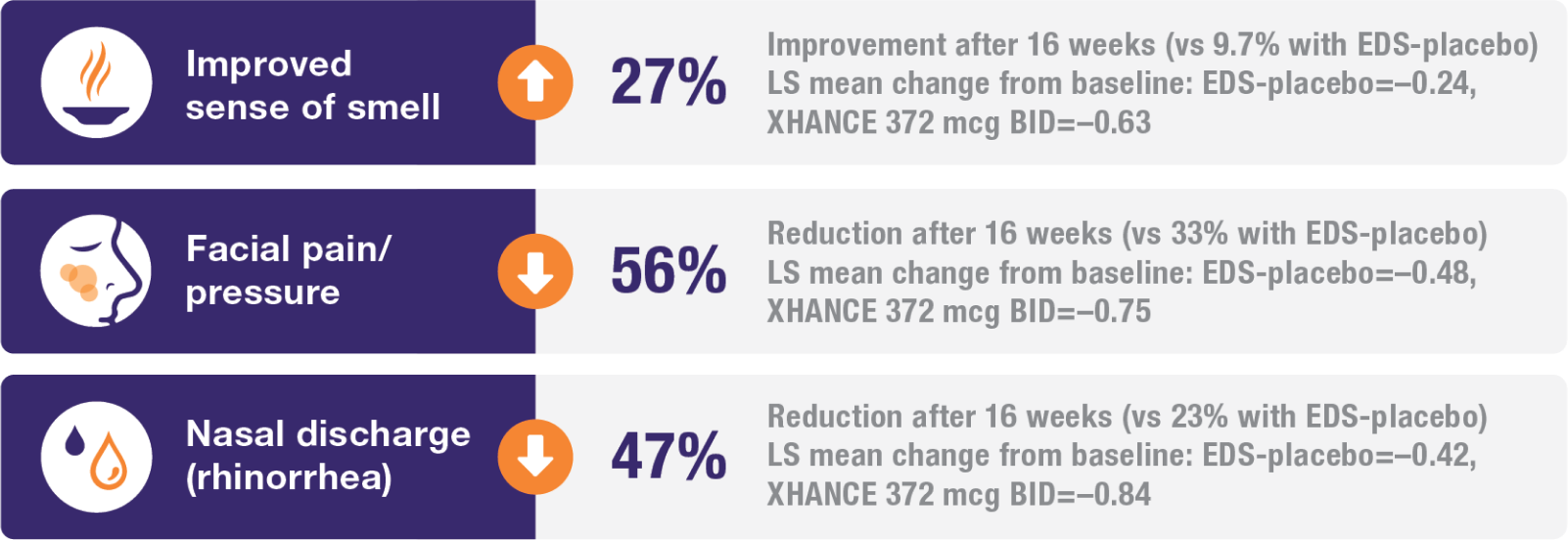
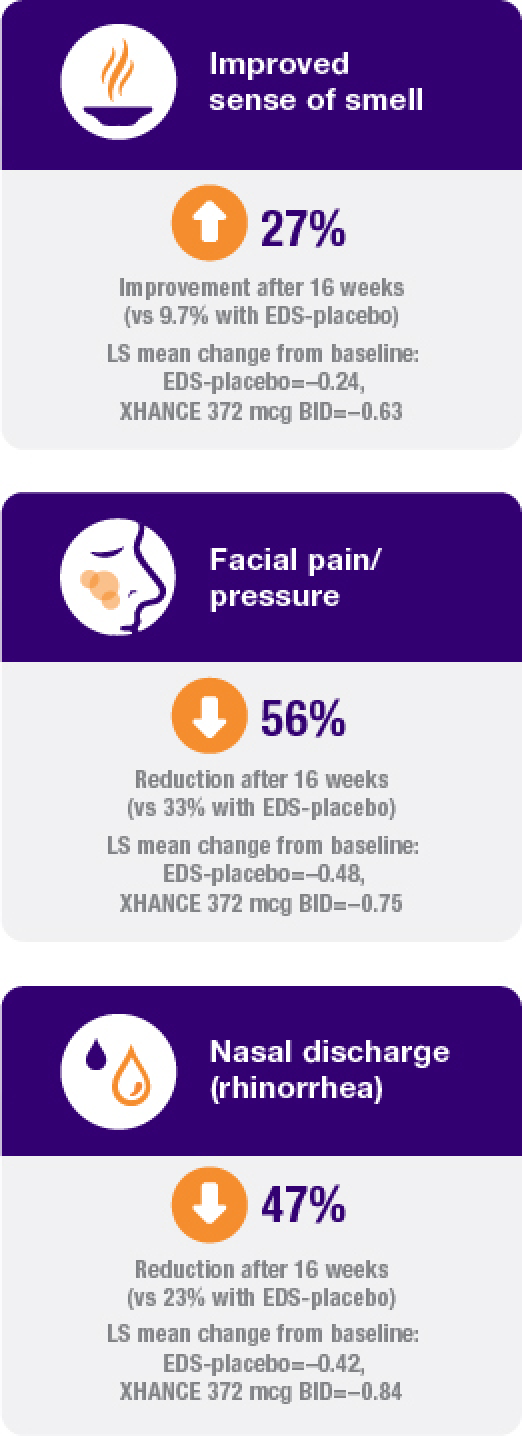
Treatment with XHANCE helped improve all 4 defining symptoms of nasal polyps.
90.6% of patients reported previous use of a topical steroid nasal spray for the treatment of nasal polyps, and 53.6% reported previous sinus surgery or polypectomy.1
NOTE: Multiplicity adjustments were not applied for secondary endpoints; therefore, results could potentially represent chance findings.
XHANCE trial design & details
XHANCE is a corticosteroid indicated for the treatment of nasal polyps in patients 18 years of age or older.XHANCE has been extensively studied in over 1500 patients.1-5
646 patients with bilateral nasal polyps evaluated for efficacy and safety for up to 24 weeks2,3
- NAVIGATE I and NAVIGATE II were double-blind, placebo-controlled trials lasting 16 weeks with an 8-week open-label extension in patients with bilateral nasal polyps and moderate to severe congestion. All patients received XHANCE 372 mcg BID during the open-label extension1-3
928 patients with chronic sinusitis with and without nasal polyps in 2 open-label trials evaluated for safety for up to 12 months1,4
- EXHANCE-3 and EXHANCE-12 were open-label studies lasting 3 months (n=705) and 12 months (n=223)1,4

In NAVIGATE I and NAVIGATE II, XHANCE was studied in a patient population in which1:
- 91% had reported previous use of a nasal steroid for the treatment of nasal polyps
- 54% reported previous sinus surgery or polypectomy
NAVIGATE I and NAVIGATE II: Phase 3 trials evaluating efficacy and safety
Two similar randomized, placebo-controlled, multicenter studies to assess XHANCE safety and efficacy (n=646)1


NAVIGATE I: EDS-placebo, n=82; XHANCE 186 mcg BID, n=80; XHANCE 372 mcg BID, n=80. NAVIGATE II: EDS-placebo, n=79; XHANCE 186 mcg BID, n=80; XHANCE 372 mcg BID, n=82.1
Coprimary endpoints1:
- Improvement in nasal congestion/obstruction at Week 4
- Reduction in bilateral polyp grade at Week 16
Secondary endpoints include2,3:
- Change from baseline in sense of smell, rhinorrhea, and facial pain or pressure
- Subject assessment of Patient Global Impression of Change (PGIC) at Week 16
- Change in Sino-Nasal Outcome Test-22 (SNOT-22) scores at Week 16
Key inclusion criteria1:
- Bilateral nasal polyps (grade 1 to 3)
- Moderate-to-severe symptoms of nasal congestion/obstruction
Details2,3,5:
- The comparator used in the pivotal clinical studies was a liquid placebo delivered with an Optinose Exhalation Delivery System (EDS)
- Patients and physicians remained blinded to initial treatment throughout the 8-week open-label extension
- Patients with history of allergic rhinitis could participate in the study provided their “season” did not coincide with the first 4 weeks of the study
- Subjects were allowed to use nonsedating antihistamines as “rescue” after Week 4 in an effort to reduce placebo dropout
EXHANCE-3 and EXHANCE-12: Phase 3 open-label studies
Data demonstrating safety for up to 1 year were also evaluated in 2 open-label studies in 928 patients with chronic sinusitis (CS) with or without nasal polyps.1,4*


Key inclusion criteria4:
- CS with or without nasal polyps*
- Experiencing ≥2 defining symptoms of CS
*XHANCE is not indicated to treat CS.
- Full Prescribing Information for XHANCE (fluticasone propionate). OptiNose US, Inc.; 2023.
- Leopold DA, Elkayam D, Messina JC, et al. NAVIGATE II: randomized, double-blind trial of the exhalation delivery system with fluticasone (EDS-FLU) for nasal polyposis. J Allergy Clin Immunol. 2019;143(1):126-134.
- Sindwani R, Han JK, Soteres DF, et al. NAVIGATE I: randomized, placebo-controlled, double-blind trial of the Exhalation Delivery System with fluticasone for chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2019;33(1):69-82.
- Palmer JN, Jacobson KW, Messina JC, et al. EXHANCE-12: 1-year study of the exhalation delivery system with fluticasone (EDS-FLU) in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2018;8(8):869-876.
- Data on file. OptiNose US, Inc.
Polyp Elimination (Grade=0) in at Least One Nostril2 — Secondary Endpoint



NOTE: Multiplicity adjustments were not applied for secondary endpoints; therefore, results could potentially represent chance findings. Furthermore, open-label results may be confounded by evaluator bias.
XHANCE trial design & details
XHANCE is a corticosteroid indicated for the treatment of nasal polyps in patients 18 years of age or older.XHANCE has been extensively studied in over 1500 patients.1-5
646 patients with bilateral nasal polyps evaluated for efficacy and safety for up to 24 weeks2,3
- NAVIGATE I and NAVIGATE II were double-blind, placebo-controlled trials lasting 16 weeks with an 8-week open-label extension in patients with bilateral nasal polyps and moderate to severe congestion. All patients received XHANCE 372 mcg BID during the open-label extension1-3
928 patients with chronic sinusitis with and without nasal polyps in 2 open-label trials evaluated for safety for up to 12 months1,4
- EXHANCE-3 and EXHANCE-12 were open-label studies lasting 3 months (n=705) and 12 months (n=223)1,4

In NAVIGATE I and NAVIGATE II, XHANCE was studied in a patient population in which1:
- 91% had reported previous use of a nasal steroid for the treatment of nasal polyps
- 54% reported previous sinus surgery or polypectomy
NAVIGATE I and NAVIGATE II: Phase 3 trials evaluating efficacy and safety
Two similar randomized, placebo-controlled, multicenter studies to assess XHANCE safety and efficacy (n=646)1


NAVIGATE I: EDS-placebo, n=82; XHANCE 186 mcg BID, n=80; XHANCE 372 mcg BID, n=80. NAVIGATE II: EDS-placebo, n=79; XHANCE 186 mcg BID, n=80; XHANCE 372 mcg BID, n=82.1
Coprimary endpoints1:
- Improvement in nasal congestion/obstruction at Week 4
- Reduction in bilateral polyp grade at Week 16
Secondary endpoints include2,3:
- Change from baseline in sense of smell, rhinorrhea, and facial pain or pressure
- Subject assessment of Patient Global Impression of Change (PGIC) at Week 16
- Change in Sino-Nasal Outcome Test-22 (SNOT-22) scores at Week 16
Key inclusion criteria1:
- Bilateral nasal polyps (grade 1 to 3)
- Moderate-to-severe symptoms of nasal congestion/obstruction
Details2,3,5:
- The comparator used in the pivotal clinical studies was a liquid placebo delivered with an Optinose Exhalation Delivery System (EDS)
- Patients and physicians remained blinded to initial treatment throughout the 8-week open-label extension
- Patients with history of allergic rhinitis could participate in the study provided their “season” did not coincide with the first 4 weeks of the study
- Subjects were allowed to use nonsedating antihistamines as “rescue” after Week 4 in an effort to reduce placebo dropout
EXHANCE-3 and EXHANCE-12: Phase 3 open-label studies
Data demonstrating safety for up to 1 year were also evaluated in 2 open-label studies in 928 patients with chronic sinusitis (CS) with or without nasal polyps.1,4*


Key inclusion criteria4:
- CS with or without nasal polyps*
- Experiencing ≥2 defining symptoms of CS
*XHANCE is not indicated to treat CS.
- Full Prescribing Information for XHANCE (fluticasone propionate). OptiNose US, Inc.; 2023.
- Leopold DA, Elkayam D, Messina JC, et al. NAVIGATE II: randomized, double-blind trial of the exhalation delivery system with fluticasone (EDS-FLU) for nasal polyposis. J Allergy Clin Immunol. 2019;143(1):126-134.
- Sindwani R, Han JK, Soteres DF, et al. NAVIGATE I: randomized, placebo-controlled, double-blind trial of the Exhalation Delivery System with fluticasone for chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2019;33(1):69-82.
- Palmer JN, Jacobson KW, Messina JC, et al. EXHANCE-12: 1-year study of the exhalation delivery system with fluticasone (EDS-FLU) in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2018;8(8):869-876.
- Data on file. OptiNose US, Inc.
![]()
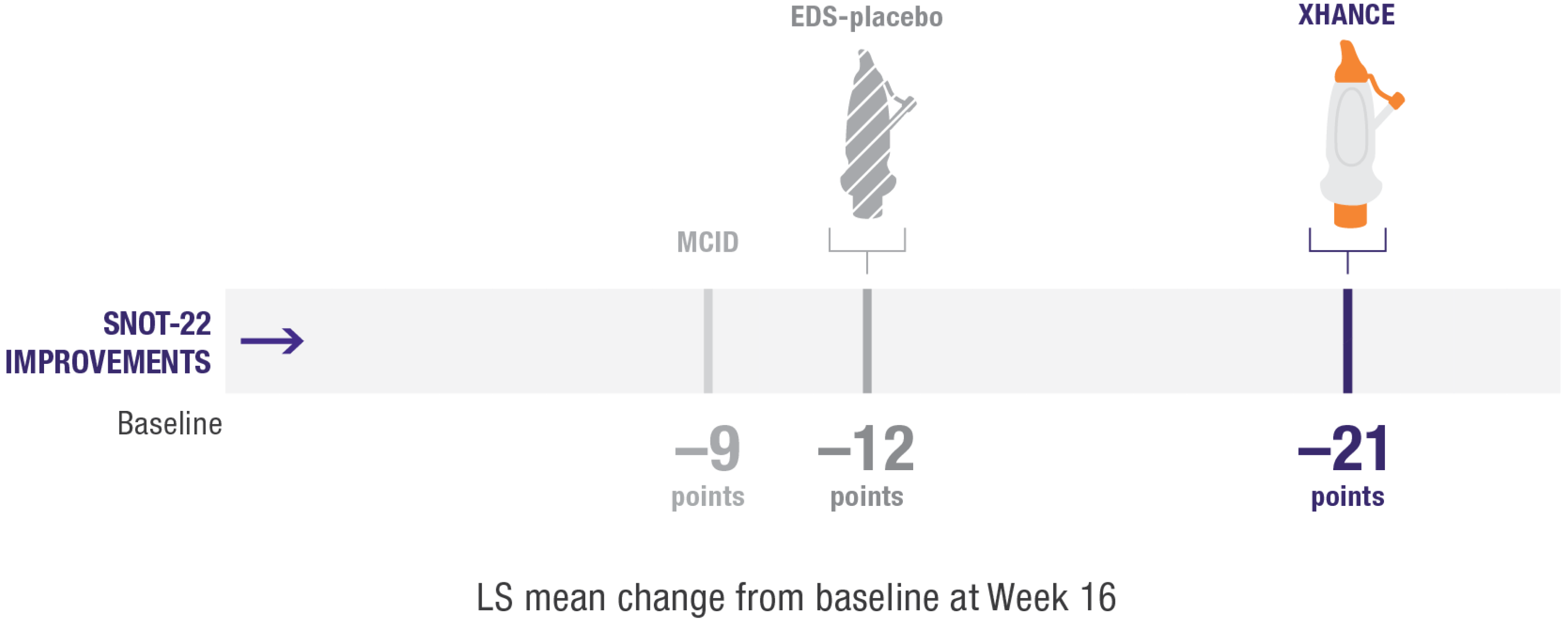
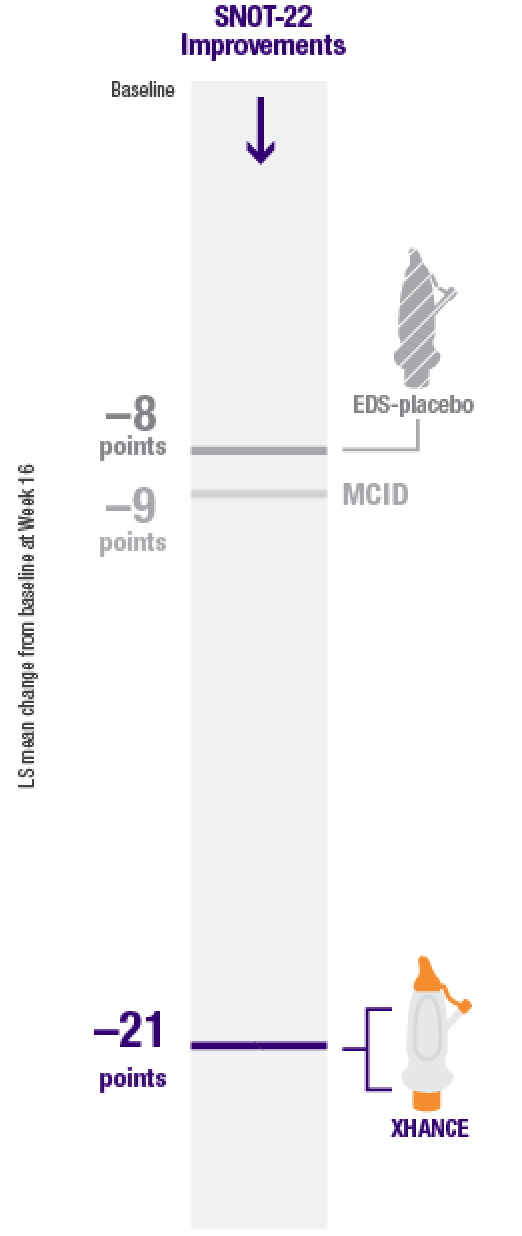
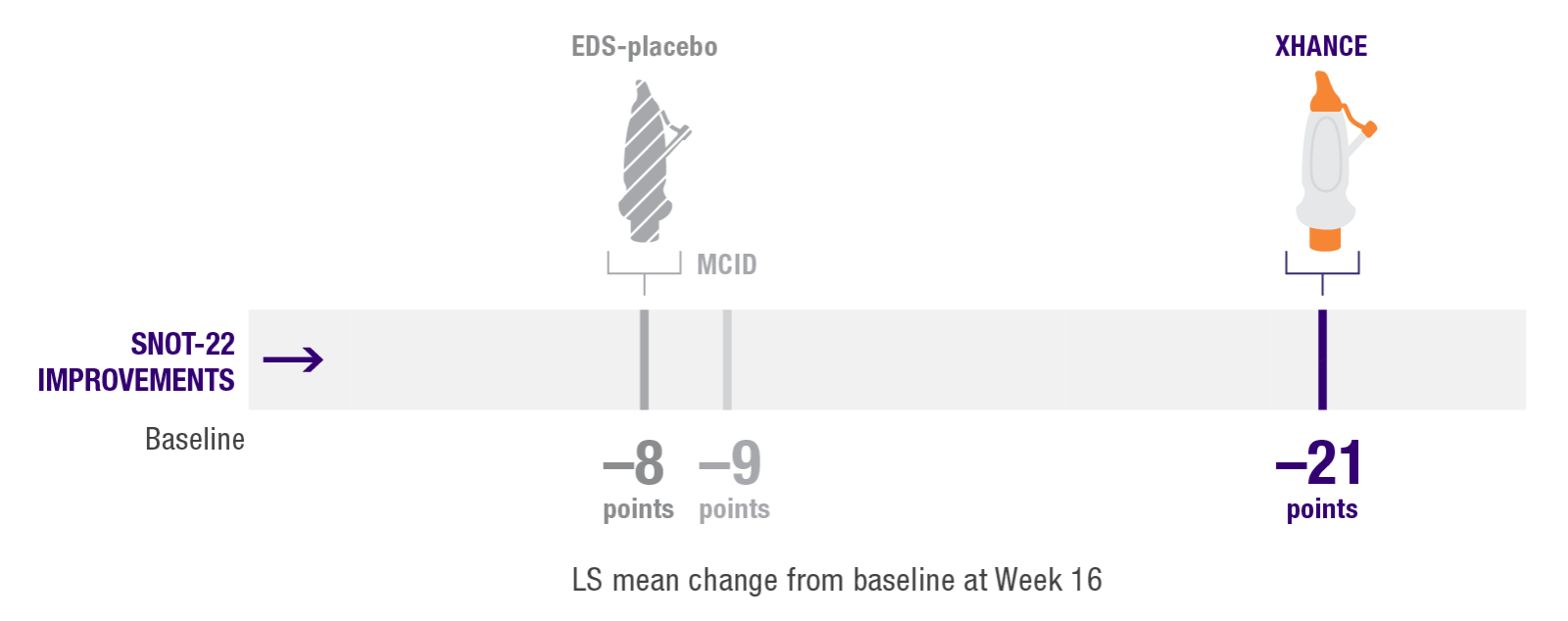
Secondary endpoint:
Improvement in quality of life as measured by SNOT-222
Improvement in SNOT-22 scores2
EDS-placebo (n=79), XHANCE 186 mcg (n=80), XHANCE 372 mcg BID (n=82).
- Mean SNOT-22 scores improved by 21 points at Week 16 in patients taking XHANCE (both the 186 mcg BID dose and the 372 mcg BID dose)2
- Minimal clinically important difference (MCID) of –9 is considered a clinically relevant improvement6,7
- In the clinical trials for XHANCE, the mean baseline SNOT-22 score for all arms was ~502,3
NOTE: Multiplicity adjustments were not applied for secondary endpoints; therefore, results could potentially represent chance findings.
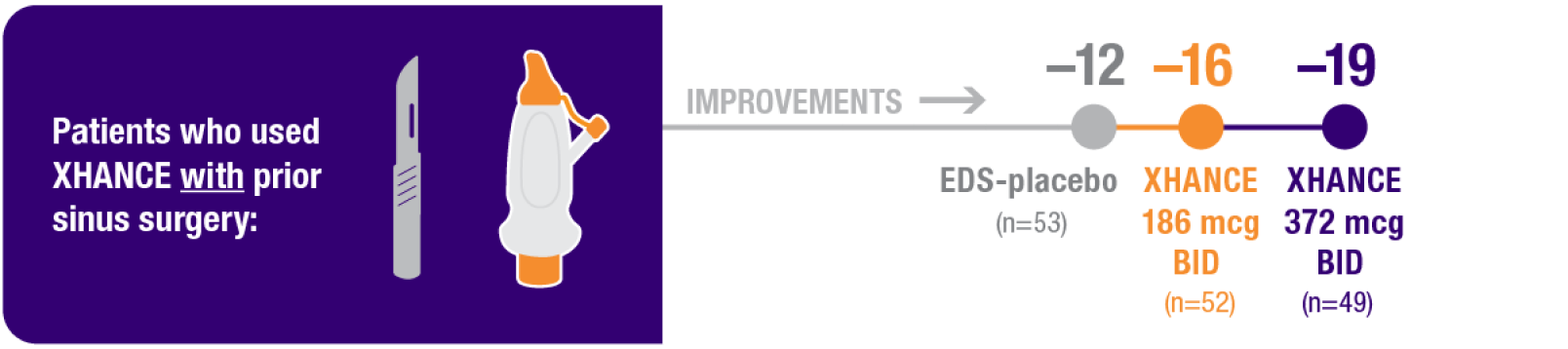
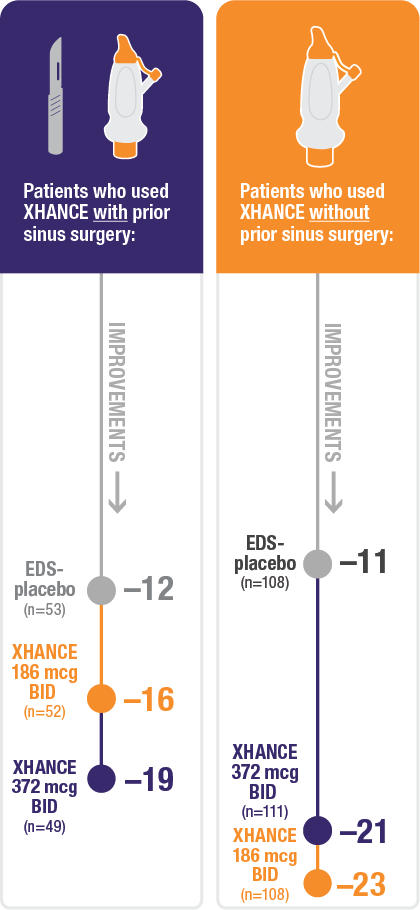
Improvement in SNOT-22 in patients with or without prior sinus surgery
Reduction in SNOT-22 score at Week 16 in the subgroups of patients with or without prior sinus surgery1
- A post hoc analysis of a pooled population from NAVIGATE I and NAVIGATE II analyzed improvement in SNOT-22 score in the subgroup of patients who had sinus surgery and those without sinus surgery prior to study entry1
- Patients were excluded from the studies if they had a history of sinus or nasal surgery within 6 months before screening2,3
Post hoc analysis of NAVIGATE I and II
Pooled subgroup data (n=481)

Baseline grade: EDS-placebo, 3.7; XHANCE 186 mcg BID, 3.8; XHANCE 372 mcg BID, 3.7.1-3
- Local Nasal Effects: epistaxis, erosion, ulceration, septal perforation, Candida albicans infection, and impaired wound healing. Monitor patients periodically for signs of possible changes on the nasal mucosa. Avoid use in patients with recent nasal ulcerations, nasal surgery, or nasal trauma until healing has occurred.
Please see additional Important Safety Information and full Prescribing Information, including Instructions for Use.
- Data on file. OptiNose US, Inc.
- Leopold DA, Elkayam D, Messina JC, et al. NAVIGATE II: randomized double-blind trial of the exhalation delivery system with fluticasone (EDS-FLU) for nasal polyposis. J Allergy Clin Immunol. 2019;143(1):126-134.
- Sindwani R, Han JK, Soteres DF, et al. NAVIGATE I: randomized, placebo-controlled, double-blind trial of the Exhalation Delivery System with fluticasone for chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2019;33(1):69-82.
Improvement in SNOT-22 in patients previously
using standard nasal steroids1
Improvements in SNOT-22 scores in the subgroup of patients reporting use of standard nasal steroids prior to study entry1
– Patients reported a mean treatment duration of 3 years with a standard nasal steroid
Post hoc analysis of NAVIGATE I and II
Pooled subgroup data (n=218)

SNOT-22 is a questionnaire for assessing symptoms, quality of life, and functioning. Patients report answers to 22 questions using a scale from 0 (“no problem”) to 5 (“problem as bad as can be”). Responses are summed, and total score ranges from 0 to 110, with a mean score of 9.3 for healthy patients.
- Local Nasal Effects: epistaxis, erosion, ulceration, septal perforation, Candida albicans infection, and impaired wound healing. Monitor patients periodically for signs of possible changes on the nasal mucosa. Avoid use in patients with recent nasal ulcerations, nasal surgery, or nasal trauma until healing has occurred.
Please see additional Important Safety Information and full Prescribing Information, including Instructions for Use.
- Senior BA, Schlosser RJ, Bosso J, Soler ZM. Efficacy of the exhalation delivery system with fluticasone in patients who remain symptomatic on standard nasal steroid sprays. Int Forum Allergy Rhinol. 2021;11(5):837-845.
- Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol. 2009;34(5):447-454.
- Le PT, Soler ZM, Jones R, Mattos JL, Nguyen SA, Schlosser RJ. Systematic review and meta-analysis of SNOT-22 outcomes after surgery for chronic rhinosinusitis with nasal polyposis. Otolaryngol Head Neck Surg. 2018;159(3):414-423.
- Leopold DA, Elkayam D, Messina JC, Kosik-Gonzalez C, Djupesland PG, Mahmoud RA. NAVIGATE II: randomized double-blind trial of the exhalation delivery system with fluticasone(EDS-FLU) for nasal polyposis. J Allergy Clin Immunol. 2019;143(1):126-134.
- Sindwani R, Han JK, Soteres DF, et al. NAVIGATE I: randomized, placebo-controlled, double-blind trial of the exhalation delivery system with fluticasone for chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2019;33(1):69-82.
XHANCE trial design & details
XHANCE is a corticosteroid indicated for the treatment of nasal polyps in patients 18 years of age or older.XHANCE has been extensively studied in over 1500 patients.1-5
646 patients with bilateral nasal polyps evaluated for efficacy and safety for up to 24 weeks2,3
- NAVIGATE I and NAVIGATE II were double-blind, placebo-controlled trials lasting 16 weeks with an 8-week open-label extension in patients with bilateral nasal polyps and moderate to severe congestion. All patients received XHANCE 372 mcg BID during the open-label extension1-3
928 patients with chronic sinusitis with and without nasal polyps in 2 open-label trials evaluated for safety for up to 12 months1,4
- EXHANCE-3 and EXHANCE-12 were open-label studies lasting 3 months (n=705) and 12 months (n=223)1,4

In NAVIGATE I and NAVIGATE II, XHANCE was studied in a patient population in which1:
- 91% had reported previous use of a nasal steroid for the treatment of nasal polyps
- 54% reported previous sinus surgery or polypectomy
NAVIGATE I and NAVIGATE II: Phase 3 trials evaluating efficacy and safety
Two similar randomized, placebo-controlled, multicenter studies to assess XHANCE safety and efficacy (n=646)1


NAVIGATE I: EDS-placebo, n=82; XHANCE 186 mcg BID, n=80; XHANCE 372 mcg BID, n=80. NAVIGATE II: EDS-placebo, n=79; XHANCE 186 mcg BID, n=80; XHANCE 372 mcg BID, n=82.1
Coprimary endpoints1:
- Improvement in nasal congestion/obstruction at Week 4
- Reduction in bilateral polyp grade at Week 16
Secondary endpoints include2,3:
- Change from baseline in sense of smell, rhinorrhea, and facial pain or pressure
- Subject assessment of Patient Global Impression of Change (PGIC) at Week 16
- Change in Sino-Nasal Outcome Test-22 (SNOT-22) scores at Week 16
Key inclusion criteria1:
- Bilateral nasal polyps (grade 1 to 3)
- Moderate-to-severe symptoms of nasal congestion/obstruction
Details2,3,5:
- The comparator used in the pivotal clinical studies was a liquid placebo delivered with an Optinose Exhalation Delivery System (EDS)
- Patients and physicians remained blinded to initial treatment throughout the 8-week open-label extension
- Patients with history of allergic rhinitis could participate in the study provided their “season” did not coincide with the first 4 weeks of the study
- Subjects were allowed to use nonsedating antihistamines as “rescue” after Week 4 in an effort to reduce placebo dropout
EXHANCE-3 and EXHANCE-12: Phase 3 open-label studies
Data demonstrating safety for up to 1 year were also evaluated in 2 open-label studies in 928 patients with chronic sinusitis (CS) with or without nasal polyps.1,4*


Key inclusion criteria4:
- CS with or without nasal polyps*
- Experiencing ≥2 defining symptoms of CS
*XHANCE is not indicated to treat CS.
- Full Prescribing Information for XHANCE (fluticasone propionate). OptiNose US, Inc.; 2023.
- Leopold DA, Elkayam D, Messina JC, et al. NAVIGATE II: randomized, double-blind trial of the exhalation delivery system with fluticasone (EDS-FLU) for nasal polyposis. J Allergy Clin Immunol. 2019;143(1):126-134.
- Sindwani R, Han JK, Soteres DF, et al. NAVIGATE I: randomized, placebo-controlled, double-blind trial of the Exhalation Delivery System with fluticasone for chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2019;33(1):69-82.
- Palmer JN, Jacobson KW, Messina JC, et al. EXHANCE-12: 1-year study of the exhalation delivery system with fluticasone (EDS-FLU) in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2018;8(8):869-876.
- Data on file. OptiNose US, Inc.
Patient Global Impression of Change (PGIC) at Week 162
Secondary endpoint:
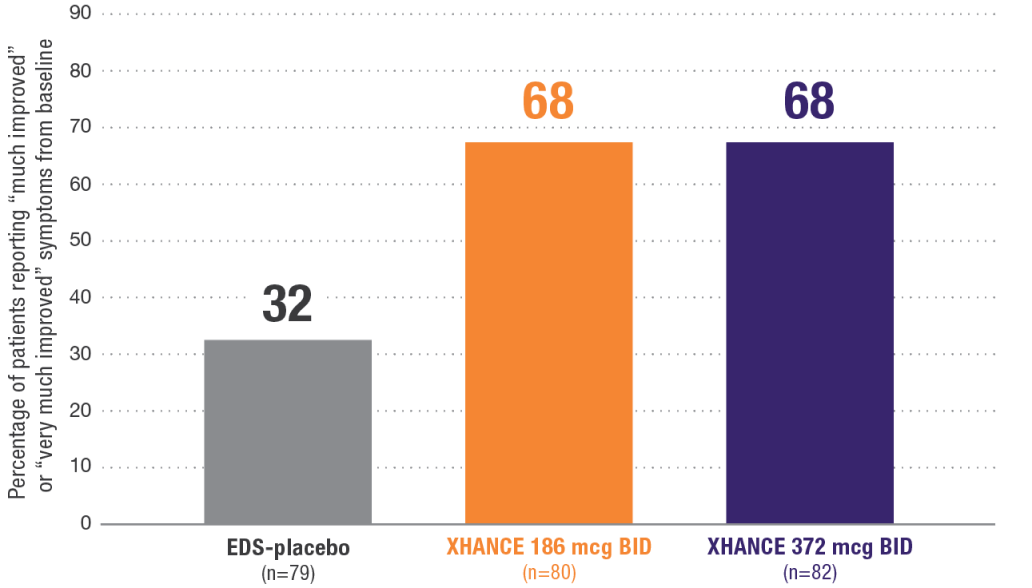

~68% of patients reported a Patient Global Impression of Change (PGIC) of “much improved” or “very much improved”2
PGIC is a commonly used method of assessing clinically important change. With PGIC, the qualitative assessment of meaningful change is determined by the patient using a 7-item scale ranging from “very much worse” to “very much improved.”
NOTE: Multiplicity adjustments were not applied for secondary endpoints; therefore, results could potentially represent chance findings.
XHANCE trial design & details
XHANCE is a corticosteroid indicated for the treatment of nasal polyps in patients 18 years of age or older.XHANCE has been extensively studied in over 1500 patients.1-5
646 patients with bilateral nasal polyps evaluated for efficacy and safety for up to 24 weeks2,3
- NAVIGATE I and NAVIGATE II were double-blind, placebo-controlled trials lasting 16 weeks with an 8-week open-label extension in patients with bilateral nasal polyps and moderate to severe congestion. All patients received XHANCE 372 mcg BID during the open-label extension1-3
928 patients with chronic sinusitis with and without nasal polyps in 2 open-label trials evaluated for safety for up to 12 months1,4
- EXHANCE-3 and EXHANCE-12 were open-label studies lasting 3 months (n=705) and 12 months (n=223)1,4

In NAVIGATE I and NAVIGATE II, XHANCE was studied in a patient population in which1:
- 91% had reported previous use of a nasal steroid for the treatment of nasal polyps
- 54% reported previous sinus surgery or polypectomy
NAVIGATE I and NAVIGATE II: Phase 3 trials evaluating efficacy and safety
Two similar randomized, placebo-controlled, multicenter studies to assess XHANCE safety and efficacy (n=646)1


NAVIGATE I: EDS-placebo, n=82; XHANCE 186 mcg BID, n=80; XHANCE 372 mcg BID, n=80. NAVIGATE II: EDS-placebo, n=79; XHANCE 186 mcg BID, n=80; XHANCE 372 mcg BID, n=82.1
Coprimary endpoints1:
- Improvement in nasal congestion/obstruction at Week 4
- Reduction in bilateral polyp grade at Week 16
Secondary endpoints include2,3:
- Change from baseline in sense of smell, rhinorrhea, and facial pain or pressure
- Subject assessment of Patient Global Impression of Change (PGIC) at Week 16
- Change in Sino-Nasal Outcome Test-22 (SNOT-22) scores at Week 16
Key inclusion criteria1:
- Bilateral nasal polyps (grade 1 to 3)
- Moderate-to-severe symptoms of nasal congestion/obstruction
Details2,3,5:
- The comparator used in the pivotal clinical studies was a liquid placebo delivered with an Optinose Exhalation Delivery System (EDS)
- Patients and physicians remained blinded to initial treatment throughout the 8-week open-label extension
- Patients with history of allergic rhinitis could participate in the study provided their “season” did not coincide with the first 4 weeks of the study
- Subjects were allowed to use nonsedating antihistamines as “rescue” after Week 4 in an effort to reduce placebo dropout
EXHANCE-3 and EXHANCE-12: Phase 3 open-label studies
Data demonstrating safety for up to 1 year were also evaluated in 2 open-label studies in 928 patients with chronic sinusitis (CS) with or without nasal polyps.1,4*


Key inclusion criteria4:
- CS with or without nasal polyps*
- Experiencing ≥2 defining symptoms of CS
*XHANCE is not indicated to treat CS.
- Full Prescribing Information for XHANCE (fluticasone propionate). OptiNose US, Inc.; 2023.
- Leopold DA, Elkayam D, Messina JC, et al. NAVIGATE II: randomized, double-blind trial of the exhalation delivery system with fluticasone (EDS-FLU) for nasal polyposis. J Allergy Clin Immunol. 2019;143(1):126-134.
- Sindwani R, Han JK, Soteres DF, et al. NAVIGATE I: randomized, placebo-controlled, double-blind trial of the Exhalation Delivery System with fluticasone for chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2019;33(1):69-82.
- Palmer JN, Jacobson KW, Messina JC, et al. EXHANCE-12: 1-year study of the exhalation delivery system with fluticasone (EDS-FLU) in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2018;8(8):869-876.
- Data on file. OptiNose US, Inc.

Review details of NAVIGATE I in the American Journal of Rhinology and Allergy.

Review details of NAVIGATE II in the Journal of Allergy and Clinical Immunology.
NOTE: These scientific publications contain additional outcome measures and other information, including exploratory endpoints, not contained in the full Prescribing Information, which are subject to important limitations.
